1. Acute Myocarditis and Transition to Fulminant Forms
Within the spectrum of acute myocarditis (AM) lies a critical subset known as fulminant myocarditis (FM). It is characterised by the occurrence of potentially life-threatening acute HF. Patients suffering from FM often experience a quick progression towards cardiogenic shock (CS) and require immediate medical interventions. Several factors are known to highlight the transition from AM to fulminant forms and might act as ’red flags’ for the attending physician. Rapid onset of symptoms, including severe HF, CS, and the occurrence of malignant arrhythmias, can be viewed as precursors of aggravating situations subsequently requiring inotropes or MCS [
3]. Furthermore, the occurrence of left bundle branch block (LBBB), premature ventricular contractions, decreased QRS amplitude, and ventricular tachycardia are reported to mark unfavourable progressions [
8]. Regarding the transition towards FM, highlighting the mentioned factors holds particularly true in the absence of ischemic or pre-existing cardiomyopathies [
9].
Epidemiological data on AM, in particular FM, appear scarce. Acute presentations seem equally distributed amongst male and female patients and appear more common in younger adults [
10]. Based on the 2019 Global Burden of Disease Report, AM has an estimated rate of 6.1 cases per 100,000 in men and 4.4 per 100,000 in women aged between 20 and 44 years [
11]. Furthermore, the prevalence of FM among patients suffering from AM was reported between 5% and 10%. Considering these estimations, the disease constitutes a rare yet highly challenging condition. Data from an international registry of 16 tertiary centres in Europe, the US, and Japan identified patients with AM and stratified them based on fulminant and nonfulminant presentations. Over the time span of 18 years, 220 patients were included. All patients presented with left ventricular systolic dysfunction, and 165 cases were classified as FM. Individuals diagnosed with fulminant forms exhibited significantly increased rates of mortality (ranging from approximately 12 to 62% under MCS,
Table 1) and heart transplantation regarding both short- and long-term outcomes [
12].
Table 1. List of studies on MCS in AM. VA-ECMO—venoarterial extracorporeal membrane oxygenation; IABPs—intra-aortic balloon pumps; HTx—heart transplant; VAD—ventricular assist device; FM—fulminant myocarditis.
| Authors |
Time Period |
Patients (n) |
Type of MCS |
Median Age |
Outcomes |
| Aoyama, N., et al. [13] |
1989–2000 |
52 |
VA-ECMO |
~48 years |
57.7% survival and return to normal life |
| Asaumi, Y., et al. [14] |
1993–2001 |
14 |
VA-ECMO |
~38 years |
71.4% were weaned and survived to discharge |
| Hsu, K.H., et al. [15] |
1994–2009 |
75 |
VA-ECMO |
~30 years |
61% survival to discharge |
| Ting, M., et al. [16] |
1994–2014 |
93 |
VA-ECMO |
~42 years |
50.1% transplant-free survival |
| Ishida, K., et al. [17] |
1995–2010 |
20 |
VA-ECMO |
~45 years |
60% survival to discharge |
| Diddle, J.W., et al. [18] |
1995–2011 |
147 |
mainly VA-ECMO |
~31 years |
61% survival to discharge (9 HTx) |
| Matsumoto, M., et al. [19] |
1995–2014 |
37 |
VA-ECMO |
~43 years |
59% successfully weaned from VA-ECMO |
| Chang, J.J., et al. [10] |
1997–2011 |
294 |
99 IABP/195 VA-ECMO |
~45/41 years |
81%/61% survival to discharge |
| Mirabel, M., et al. [20] |
2002–2009 |
35 |
VA-ECMO |
~38 years |
68.6% survival to discharge |
| Wu, M.Y., et al. [21] |
2003–2010 |
16 |
VA-ECMO |
N/A |
87.5% survival to discharge |
| Beurtheret, S., et al. [22] |
2005–2009 |
14 |
VA-ECMO |
N/A |
65% survival to discharge |
| Chou, H.W., et al. [23] |
2006–2018 |
88 |
VA-ECMO |
~42 years |
46.6% successful weaning and discharge |
| Tadokoro, N., et al. [24] |
2006–2020 |
70 |
VA-ECMO cent. 48/periph.22 |
~44/50 years |
62%/95% weaning from VA-ECMO (total cohort survival 5 years: 76%) |
| Mody, K.P., et al. [25] |
2007–2013 |
11 |
3 VA-ECMO/8 Bi-VAD |
~48 years |
73% survival to discharge (2 permanent VAD) |
| Lorusso, R., et al. [26] |
2008–2013 |
57 |
VA-ECMO |
~38 years |
72% survival to discharge |
| Saito, S., et al. [27] |
2009–2015 |
25 |
23 VA-ECMO/2 t-VAD |
~39 years |
83.3% survival to discharge (6 permanent VAD) |
| Annamalai, S.K., et al. [28] |
2009–2016 |
34 |
Impella (2.5, CP, 5.0, or RP) |
~42 years |
61.8% survival to discharge (15 weaned, 5 transferred, 1 HTx) |
| Nunez, J.I., et al. [29] |
2011–2020 |
850 |
VA-ECMO |
~41 years |
65.1% survival to discharge |
| Danial, P., et al. [30] |
2015–2018 |
47 |
VA-ECMO |
~46 years |
37.9% survival to discharge |
| Tonna, J.E., et al. [31] |
2020–2021 |
88 |
VA-ECMO |
~48 years |
49% survival to discharge (FM + COVID-19) |
| Ammirati, E., et al. [32] |
2020–2021 |
10 |
IABP/VA-ECMO |
~38 years |
78.5% survival after 120 days (FM + COVID-19) |
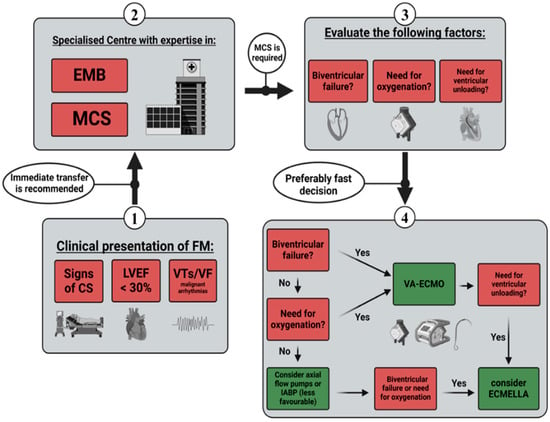
2. Indication and Timing of Short-Term MCS
Evidence-based recommendations regarding MCS in FM are scarce. The use of short-term options, including ECMO, intra-aortic balloon pumps (IABPs), and axial flow pumps, has been reported [
15,
28]. However, there is no clear consensus on the appropriate timing for initial MCS in the particular setting of myocarditis. Therapeutic decisions are often based on the degree of haemodynamic stability, metabolic and organ function, local procedures, and the general availability of mechanical support systems. Within an expert consensus document, Ammirati et al. summarised contemporary evidence and identified factors associated with a high probability of MCS requirement. The authors emphasised the clinical presence of cardiogenic shock, a left ventricular ejection fraction (LVEF) of less than 30%, and life-threatening arrhythmias as significant predictors of MCS necessity. In the presence of these factors, referral to a specialised centre, EMB, and temporary MCS are to be considered [
9]. A position statement by the working group on myocardial and pericardial diseases (European Society of Cardiology, ESC) outlined similar recommendations. Patients displaying life-threatening manifestations of HF should be transferred to specialised units capable of extensive haemodynamic monitoring and EMB. Furthermore, in patients with haemodynamic instability, MCS systems may serve as a bridge to recovery or heart transplantation [
2].
More recent evidence has been reviewed by the current ESC guidelines for the diagnosis and treatment of acute and chronic heart failure (published in 2021 and updated in 2023). Although not specific to FM, general recommendations are given for acute HF (AHF) and CS [
40,
41]. The guidelines recommend using MCS strategies selectively in specialised centres with multidisciplinary expertise regarding the implantation and management of circulatory support systems [
42]. A ‘standardised team-based approach’ including early MCS implantation and close monitoring of invasive haemodynamics, laboratory markers indicating end-organ damage, and serial lactate measurements was reported to potentially improve outcomes [
43,
44]. The IABP-SHOCK-II trial revealed no tangible differences in 30-day mortality comparing optimal medical therapy and IABPs in CS patients following acute coronary syndromes with early revascularisation [
45]. While not being investigated in all aetiologies of shock, the use of IABPs as a bridging option was assigned a class of recommendation IIb and a level of evidence C in patients with CS (class of recommendation III, level of evidence B in patients following myocardial infarction). Despite these recommendations, IABPs might improve ventricular unloading and coronary circulation. Other short-term MCS systems were evaluated more favourably in this context, with a class of recommendation IIa and a level of evidence C [
40]. Moreover, the ESC guidelines particularly highlight the use of VA-ECMO in FM and other conditions causing pronounced CS [
46]. Although only tested in infarct-related CS, the ECLS-SHOCK trial may question the efficacy of early ECMO implantation in unselected patient cohorts if there is no definitive therapy after bridging [
47]. Considering the potentially reversible nature of FM, temporary MCS strategies should be prioritised in the early stages over permanent solutions.
An analysis by Pahuja et al. uncovered trends in the epidemiology of myocarditis, CS, and the associated use of MCS in the United States from 2005 to 2014. They reported an increasing incidence in myocarditis paired with a drastically increased prevalence in CS (6.94% in 2005 vs. 11.99% in 2014) and required MCS (4.5% in 2005 vs. 8.6% in 2014). Contrary to these findings, in-hospital mortality remained unchanged (4.43% of total admissions over the study period), which might reflect the benefits of increased MCS utilisation. Moreover, within the observed time period, the usage of all MCS systems except IABPs increased significantly [
48]. This particular finding can be interpreted in favour of short-term MCS, like ECMO or axial flow pumps, and may support the current ESC guideline recommendations regarding bridging strategies in CS [
40]. According to available evidence in FM, the median time from the onset of AHF to ECMO implantation was reported between 13 and 15 h [
14,
18]. Previous studies on other aetiologies of CS suggested better outcomes with earlier implantation [
49,
50,
51]. Axial flow pumps can serve as a treatment option for patients experiencing isolated left ventricular failure and for those without the need for supplemental extracorporeal oxygenation (or decarboxylation) support. Annamalai et al. conducted a study on the initial management of patients suffering from FM utilising Impella™ devices. Of the 34 patients included, 10 individuals required additional MCS, whereas complete recovery was observed in 15 cases without further support [
28]. Randomised controlled trials (RCTs) comparing different MCS strategies in the early management of FM are lacking. Therefore, only assumptions and eminence-based opinions regarding the efficacy of various MCS systems are available. Based on the highlighted recommendations and supporting evidence, the researchers created a diagram proposing a management sequence for short-term MCS in FM patients (
Figure 1).
Figure 1. Management sequence for short-term MCS. CS—cardiogenic shock; LEVF—left ventricle ejection fraction; VT—ventricular tachycardia; VF—ventricular flutter; EMB—endomyocardial biopsy; MCS—mechanical circulatory support; IABPs—intra-aortic balloon pumps; VA-ECMO—venoarterial extracorporeal membrane oxygenation.
3. Outcomes in Fulminant Myocarditis with Short-Term MCS
Analysing the available evidence for short-term MCS in FM, consistent patterns appear to be present. Most patients were young to middle-aged adults with a median age of 31 to 51 years. VA-ECMO was most commonly used, with reported survival rates ranging from 38% to 87.5% at discharge (outlined in
Table 1). According to the majority of the displayed studies, the survival rates for FM are generally higher than 60%. This indicates better outcomes in comparison to other causes of CS. In a study of 850 patients with suspected AM, Nunez et al. found that the hospital discharge rate of 65.1% was significantly higher than the 41% in an all-comer collective [
29]. This finding underlines the importance of short-term MCS in haemodynamically unstable patients and emphasises the potentially reversible nature of the disease. Outcomes in FM associated with COVID-19 seem comparable to other aetiologies. Tonna et al. reported a 49% rate of survival to discharge, whereas Ammirati et al. found a survival rate of 78.5% after 120 days [
31,
32]. The data regarding IABPs and Impella™ show promising results in the context of survival and weaning success [
10,
28,
32]. However, an underlying selection bias towards less critical patients without the need for additional oxygenation and biventricular failure cannot be ruled out. Moreover, in light of the latest recommendations for CS, IABPs can be viewed as less favourable compared to other short-term MCS systems [
40]. Comparing central versus peripheral VA-ECMO, Tadokoro et al. highlighted better outcomes in patients with peripheral cannulation [
24]. Whether this finding was driven by procedural differences or is attributed to the selection of patients without the need for ventricular unloading remains unanswered.
4. Ventricular Unloading in Short-Term MCS
Although VA-ECMO implantation may be necessary for haemodynamic stabilisation in critically ill patients, it invariably increases left ventricular afterload. Without proper venting strategies, the resulting dilation of the left ventricle (LV) may cause pulmonary oedema as well as impaired myocardial regeneration due to haemodynamic and inflammatory pathomechanisms [
52]. These adverse changes may be of particular importance in FM, as it constitutes a disease primarily driven by inflammation. Therefore, reducing wall stress through unloading may subsequently reduce the inflammatory response and improve ventricular recovery [
53]. Implantation of axial flow pumps can potentially achieve this goal, and multiple case reports highlighted positive results in the short-time use of LV-Impella™ in FM [
54,
55,
56]. Compared to different surgical venting strategies utilising VA-ECMO and cannulation of the left atrium (LA) or the LV, percutaneous Impella™ implantation may prove to pose a lower risk of bleeding or the occurrence of thromboembolism. Comparing Impella™ and ECMO treatment in patients with CS, Lamarche et al. reported comparable rates of 30-day mortality and hospital discharge. However, arterial thrombus formation and the requirement for blood products were found to be statistically less frequent in patients receiving Impella™ [
57]. Overall, VA-ECMO and Impella™ treatment for FM were found to result in similar survival to discharge rates of approximately 60% [
10,
28].
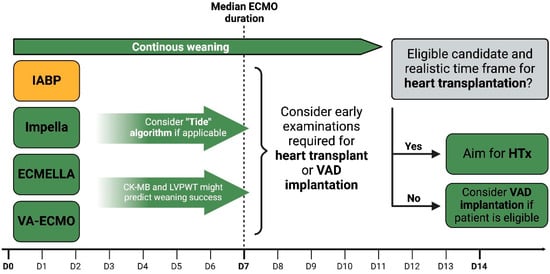
5. Weaning Strategies and Transition to Long-Term MCS
FM represents a condition with potential myocardial recovery, and short-term MCS systems should be used initially. However, questions regarding potential weaning strategies and the optimal time for transition to long-term MCS or heart transplantation arise. There are currently no guidelines or recommendations that are based on sufficient evidence. Despite the associated procedural risks and potential long-term complications, timely transplantation represents a feasible option for patients on MCS without significant weaning progress. In their study, Hsu et al. enrolled 75 adults suffering from FM who required VA-ECMO. Three patients received successful heart transplantation and survived to discharge [
15]. In a study conducted by Ting et al., six patients with previous MCS underwent heart transplantation. Four of these patients survived to discharge [
16]. Although heart transplantation appears to be a viable option in FM, not all patients in need of protracted haemodynamical support are eligible candidates for the procedure. Additionally, global and regional shortages of donor organs create the requirement for alternatives [
66].
There are currently no clear recommendations regarding the duration of short-term MCS and the exact point at which patients should be transitioned to long-term MCS or heart transplantation. In their systematic review, Uil et al. reported a median ECMO support period of 6–7 days in patients suffering from refractory CS [
68]. In a study of paediatric patients, Lee et al. found a high likelihood of transitioning to long-term treatment options if no recovery was observed within two weeks [
69]. In the previously mentioned study by Hsu et al., patients displayed a median ECMO duration of 7 ± 5 days [
15]. The previously reported evidence seems to favour early transplantation over the implantation of long-term MCS. However, not all patients are eligible candidates, and the shortage of donor organs paired with long waiting periods makes VAD systems a situational but viable choice. There are recent publications demonstrating the effectiveness of VAD implantation in patients suffering from FM [
19,
27]. In most of the reported cases, a left ventricular assist device (LVAD) was utilised and served as a bridge to transplant, recovery, or destination therapy. However, selected patients might require a bilateral ventricular assist device (Bi-VAD) [
70]. Jaroszewski et al. even reported a patient with FM who underwent ECMO and was bridged to temporary Bi-VAD, followed by permanent VAD systems, and eventually, recovery [
71]. Based on the highlighted publications, the researchers propose a decision diagram for transitioning FM patients from short-term to long-term MCS systems or heart transplantation (
Figure 2).
Figure 2. Transitioning from short-term to long-term MCS systems or heart transplantation. IABPs—intra-aortic balloon pumps; VA-ECMO—venoarterial extracorporeal membrane oxygenation; LVPWT—left ventricular posterior wall thickness; HTx—heart transplantation; VAD—ventricular assist device.
6. MCS and Additional Treatment in Lymphocytic Myocarditis
Lymphocytic myocarditis (LM) represents a subtype of myocarditis characterised by lymphocytic infiltration of the myocardium [
72]. EMB should be performed to confirm the diagnosis and to distinguish between FM and inflammatory aetiologies characterised by infiltration of other cell lines. The clinical presentation ranges from mild symptoms to cardiogenic shock and life-threatening ventricular arrhythmias [
73]. Viral infections are purported to be the most common cause of LM and can be detected in 30–40% of the affected patients [
1]. Beyond the direct viral impact, cardiac injury may also emanate from an amplified immunological response termed molecular mimicry. This process involves the immune system erroneously targeting cardiac cells due to antigenic resemblance, thereby inducing myocardial damage [
74]. Fulminant forms may require MCS systems as a bridge to recovery or long-term options, such as heart transplantation or VAD implantation. Particularly, rapid-onset variants may profit from immediate haemodynamic support, as spontaneous myocardial recovery was reported in some of these cases [
75]. Furthermore, the MCS management of patients with fulminant LM should follow the discussed recommendations for FM and CS. Due to the inflammatory aetiology of the disease, multiple therapeutic approaches have been tested in the past. Particularly, the addition of corticosteroids and other anti-inflammatory agents were utilised with varying degrees of success. While therapy with prednisone alone did not lead to significant changes, combination with azathioprine resulted in improved myocardial recovery [
76]. However, most of the reported studies used LVEF as an endpoint, but robust data on clinical outcomes, such as mortality, are lacking. Thus, there are no clear recommendations for the use of corticosteroids or immunosuppression in addition to general measures of haemodynamic stabilisation. Nonetheless, those substances are often used in clinical practice and may be viewed as an eminence-based therapeutic approach.
7. MCS and Additional Treatment in Giant Cell Myocarditis
Giant cell myocarditis (GCM) constitutes a rare but often fatal subset of FM [
77]. This form predominantly affects young and middle-aged adults and presents with AHF as well as ventricular arrhythmias [
78]. Histological findings show multifocal inflammatory infiltrates, including lymphocytes and multinucleated giant cells, which are typically located at the edge of the lesions. Due to this distinct histological presentation, EMB plays a crucial role in the diagnosis of GCM. However, several factors can impede the reliability of the procedure. Early samples may turn out negative, as giant cells typically appear after 7–14 days [
79]. Furthermore, right ventricular EMB may be prone to the occurrence of sampling errors. Therefore, multiple biopsies, including samples of the LV, are often necessary for a definitive diagnosis of the disease [
80]. Considering these implications, short-term MCS might confer a crucial time window of haemodynamic stability needed for proper diagnosis. Additionally, it is often used as a bridge to heart transplantation, which appears to be a viable, if not the most beneficial, long-term option for GCM [
81]. Since the condition often affects both ventricles, biventricular MCS is required more often before transplantation in comparison to patients with cardiomyopathies caused by other aetiologies [
82,
83]. In addition to utilising haemodynamic support systems, early therapy with immunosuppressive agents is recommended [
3]. Patients treated with cyclosporine, corticosteroids, and with or without an anti-CD3 antibody displayed a high survival rate after one year [
84]. Due to the adverse side effects of anti-CD3 antibodies, later approaches used triple immunosuppressive therapy, including corticosteroids, cyclosporine, and azathioprine or mycophenolate mofetil [
80,
85]. A recent analysis highlighted the clear survival benefits of early immunosuppression compared to prior or exclusive treatment with MCS systems [
86]. In conclusion, MCS systems are crucial for the haemodynamic stabilisation of patients suffering from fulminant GCM. However, early immunosuppressive therapy is essential to improve transplant-free survival or even achieve myocardial recovery [
87].
8. MCS and Additional Treatment in Eosinophilic Myocarditis
Eosinophilic myocarditis (EM) is characterised by eosinophilic infiltration of the myocardium [
88]. Amongst others, the condition has been linked to hypersensitivity reactions, hypereosinophilic syndromes, autoimmune disorders, infections, and active malignancies [
89,
90,
91,
92,
93]. In a meta-analysis, Brambatti et al. characterised patients with histologically proven EM. They found that the median age was 41 years, 75.9% of them had peripheral blood eosinophilia, and there was a 22.0% correlation with asthma [
94]. One of the most severe forms of EM is known as acute necrotising eosinophilic myocarditis (NEM). Although the condition is considered rare, it is associated with the rapid onset of CS, high rates of mortality, and the necessity of heart transplantation [
95]. Similar to other aetiologies, such as GCM, EMB plays a crucial role in the diagnosis and further therapeutic management of EM and NEM. Notably, the most common cause of acute NEM appears to be drug hypersensitivity [
3]. In both regards, short-term MCS may be of particular importance in providing haemodynamic stability until the diagnosis is made or potentially triggering medication can be stopped. There have been reports of ventricular thromboembolism occurring in individuals suffering from NEM [
96,
97]. This procoagulant potential must be considered while planning MCS strategies and managing anticoagulation. In contrast to GCM, high doses of corticosteroids appear effective in NEM [
98,
99]. Further approaches included the addition of mycophenolate mofetil and azathioprine [
100,
101]. There are multiple cases in which immunosuppression was administered, and MCS systems served as a bridge to recovery [
100,
102].