Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Enzymes play an important role in numerous natural processes and are increasingly being utilized as environmentally friendly substitutes and alternatives to many common catalysts. Both horseradish peroxidase (HRP) and laccase are most often utilized for the formation of enzyme aggregates due to their ability to rapidly oxidize phenols, generating phenoxy radicals which undergo radical coupling to form biphenyl or phenyl ether linkages.
- “green” chemistry
- enzymes
- laccase
1. Laccase Catalysts in Nature
Laccases (benzenediol:oxygen oxidoreductase, EC 1.10.3.2) belong to a family of multicopper core oxidases containing a minimum of four copper atoms at their active site, classified as either Type 1 (T1), Type 2 (T2), or binuclear Type 3 (T3) Cu centers [1]. First discovered in 1883 by Hikorokuro Yoshida as a component of lacquer sap from Rhus vernicifera [2], a broad variety of laccases have since been isolated, with particular interest in those derived from fungal sources due to their ease of production, low cost, and high redox potential. Characterized by their broad substrate specificity, laccases are capable of catalyzing one-electron oxidations of a myriad of organic and inorganic substrates, although they are best known for their oxidation of phenols with the concomitant reduction in oxygen.
Being found in fungi, plants, and microorganisms, laccase has many roles in nature (Table 1). It is perhaps most well-known for its function in numerous fungi, where it catalyzes the degradation of lignin and humic acid, most probably employing naturally occurring radical mediators such as syringaldehyde and vanillin [3]. Laccase has also been suspected to play a major role in the synthesis of melanin-like pigments, where phenol and catechol derivatives are oxidized to strongly absorbing quinones [4]. Similar to fungal sources, laccases found in bacteria serve to catalyze the reactions involved in the degradation of lignin and other phenols, in addition to the synthesis of pigments. In bacteria, however, laccase also plays the unique role of assisting in the resistance to spores and pathogenesis, most likely by catalyzing the oxidation of toxic compounds [5].
In distinction to both fungal and bacterial sources, laccase in plants has been found to play a primary role in lignin synthesis by catalyzing the polymerization of monolignols accounting for the lignin component of plant cell walls, and is therefore of interest for the synthesis of synthetic lignin and lignin-like polymers [6]. Analogous to plants, insects utilize laccase as a catalyst in the development of their cuticle. This process occurs through the laccase oxidation of catechols to their corresponding quinones which can crosslink proteins of the cuticle, allowing for its hardening [7].
Table 1. Laccases: sources, molecular mass, and catalytic function in nature.
| Source | Molecular Mass (kDa) | Natural Function | Reference | |
|---|---|---|---|---|
| Fungal | Scytalidium thermophilum | 82 | Lignin degradation Pigment formation |
[8] |
| Rhizoctonia solani | 70–85 | Lignin degradation Pigment formation |
[8] | |
| Trametes versicolor | 58 | Lignin degradation Pigment formation |
[9] | |
| Bacterial | Strepotomyces antibioticus | 67.5 | Phenoxazinone synthesis | [10] |
| Bacillus subtilis | 65 | Spores pigmentation UV and H2O2 resistance |
[11] | |
| Pseudomonas syringae | 72 | Copper resistance | [12] | |
| Plant | Acer pseudoplatanus | 59.9 | Lignin Synthesis | [13] |
| Malus domestica | 74 | Lignin Synthesis | [14] | |
| Rhus vernicifera | 110 | Lignin Synthesis | [15] | |
| Insect | Manducta sexta | 220–280 | Cuticle Scleritization | [16] |
| Sarcophaga bullata | 90–100 | Cuticle Scleritization | [17] | |
| Periplaneta americana | 185 | Cuticle Scleritization | [18] | |
2. Laccase Structure and Catalytic Pathway
Although heavily dependent on the source, laccases most commonly possess a molecular mass of 60–70 kDa and can be divided into three nearly homogenous domains of β–barrel type structures which surround the active site at the center of the enzyme (D1–3, Figure 1) [19]. Laccases are further composed of covalently linked glycosidic regions, constituting between 10–45% of the enzyme’s mass (GL1–3, Figure 1) [19]. These glycosidic regions serve to protect the enzyme from thermal inactivation, proteolytic attack, and solvent-induced denaturation [20]. The substrate binding pocket in the interior of laccase is surrounded by hydrophobic fragments and histidine/aspartic acid/glutamic acid residue located nearby. This particular residue participates in hydrogen bonding with substrates containing –OH or –NH2 groups, assisting in deprotonation [21]. This enzyme structure provides the optimal catalytic activity of laccase between 50–70 °C and an optimal pH varying greatly with the substrate [22].
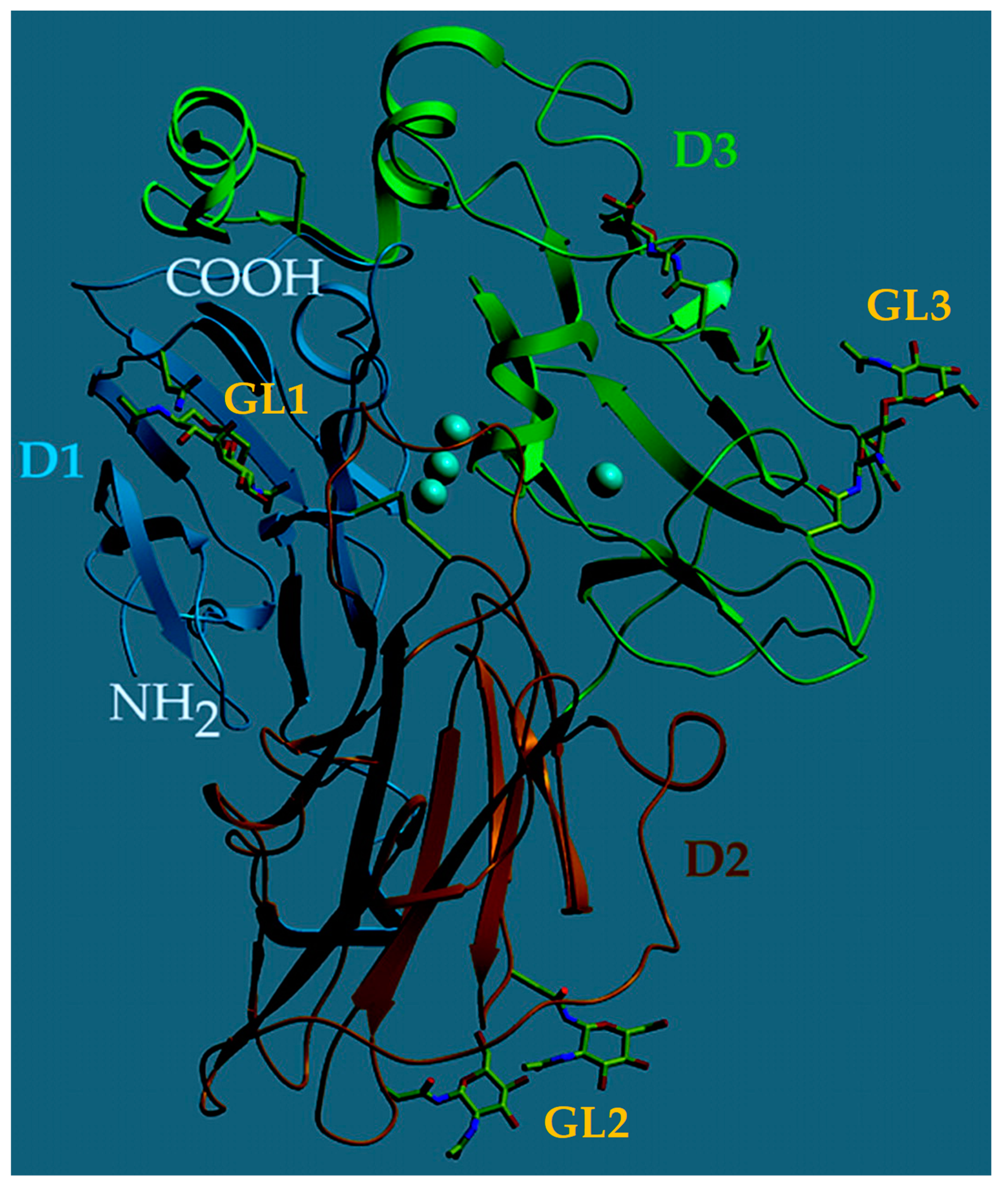
Figure 1. Three-dimensional structure of Trametes versicolor laccase. The protein domains (D1–3) are shown in dark blue, red, and green; the four Cu atoms are depicted in light blue and the glycoside anchoring sites (GL1–3) are marked in yellow. Modified from [19]. This work is licensed under CC BY.
The catalytic mechanism of laccase has been thoroughly investigated through electron paramagnetic resonance spectroscopy and X-ray crystallography, allowing for the near complete elucidation of the enzyme active site function [23]. Of primary importance for understanding the laccase mechanism of action is the elucidation of the role T1, T2, and binuclear T3 copper centers play in the process.
Investigation of the active site has revealed that the T1 site is ligated by two nitrogen atoms in histidine moieties and a thiolate residue on the enzyme. This site serves as a long-range intramolecular electron shuttle, directing electrons from the substrate to the T2 and T3 copper atoms, and is therefore the position of substrate oxidation. The two copper atoms of the T3 site are ligated by three histidine groups each and are bridged by a hydroxide anion. The T2 site is ligated by an additional two histidine residues and a water molecule. Catalysis is initiated through the oxidation of the substrate at the T1 copper site via one electron transfer. Substrates containing either hydroxyl or amine groups are often activated by the enzyme through deprotonated aspartic acid groups neighboring the active site, decreasing the strength of O–H or N–H bonds, and consequently facilitating oxidation. Electrons are then shuttled from the T1 copper to the T2 and T3 atoms, where molecular oxygen is bound and able to accept the transported electrons. Altogether, four single electron transfers are conducted to reduce one molecule of oxygen.
The KM values are also source-dependent and vary between 11 and 20 μmol.
2.1. Radical Mediators in Laccase Catalysis
While laccases have an inherently broad substrate specificity, the array of substrates capable of undergoing induced oxidation is greatly increased by the addition of catalytic amounts of radical mediators [24]. Such mediators include 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), 1-hydroxybenzotriazole (HOBT), N-hydroxyphthalimide (Figure 2), and other nitroxide-based mediators [24]. The electron-rich naphthalimide (HONI) has also been successfully employed for the enhanced oxidation of benzo-α-pyrene in water mediated by Trametes versicolor laccase regio-electively modified with amphiphilic linear-dendritic block copolymers [25]. The notable advantage of this enzyme–copolymer complex was that both the mediator and the polyaromatic hydrocarbon are highly hydrophobic but could selectively be bound by the dendritic blocks in the vicinity of the enzyme active site and facilitate/undergo oxidation. Nitroxide-based radical mediators most often function as electron shuttles and are primarily applied in reactions where the substrate is either incapable of fitting into the enzyme active site or unable to reach the active site due to spatial limitations in substrate diffusion. The electron shuttle mechanism by which these mediators act is widely accepted to occur via the laccase oxidation of the mediator’s N-OH group, producing nitroxide radical N–O● (Scheme 1). The resultant radical migrates from the enzyme active site to the substrate, where it can abstract a hydrogen radical, oxidizing the substrate and regenerating the mediator [24].
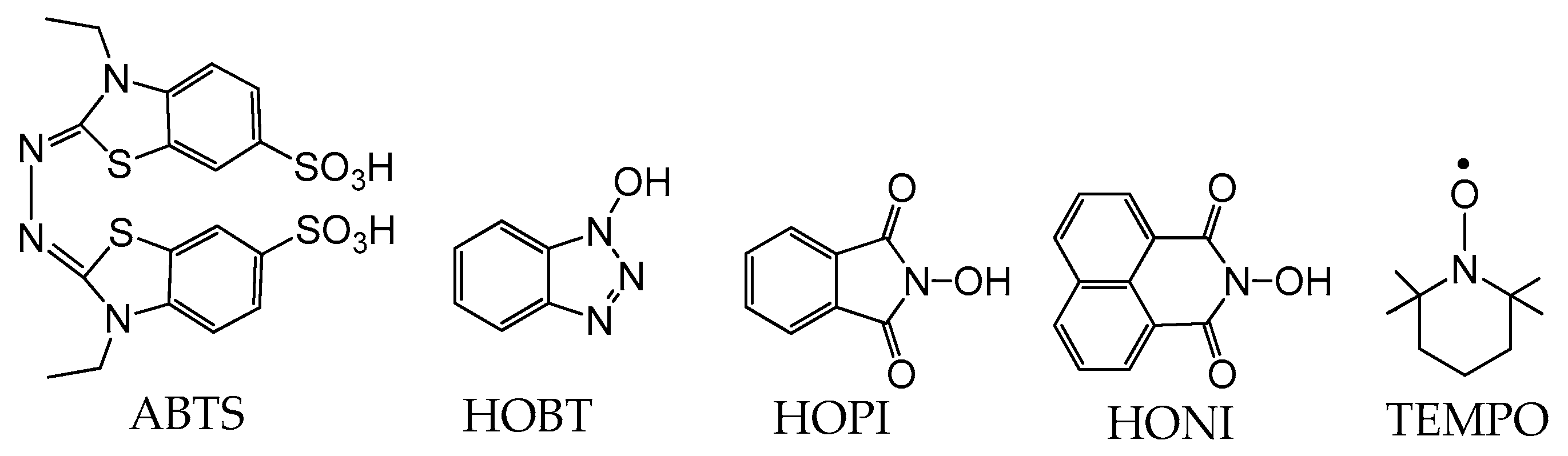
Figure 2. Selected mediators. 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid), ABTS; 1-Hydroxybenzotriazole, HOBT; N-hydroxyphthalimide, HOPI; N-hydroxynaphthalimide, HONI and 2,2,6,6-tetramethylpiperidine-N-oxyl, TEMPO.
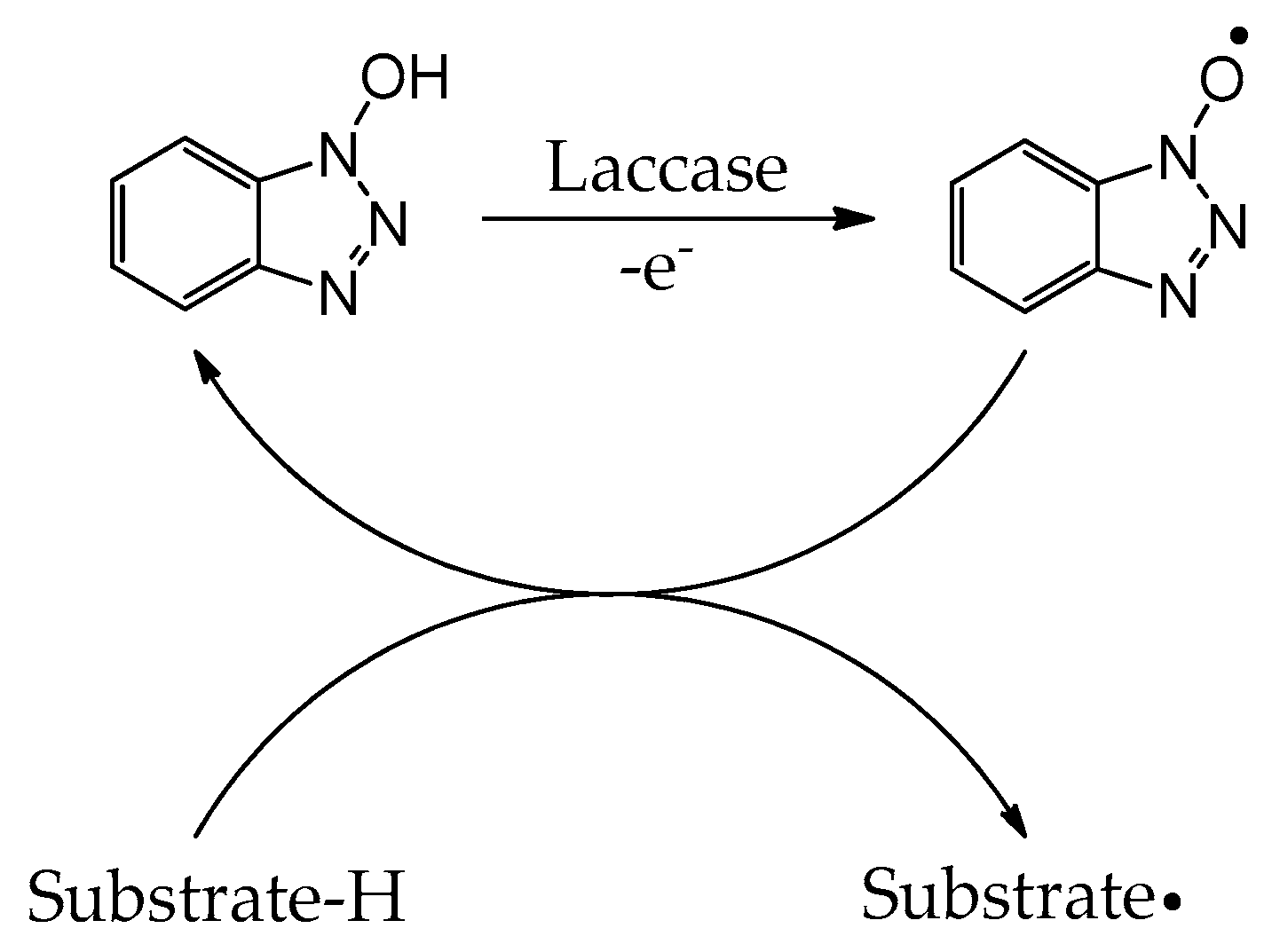
Scheme 1. Oxidation of HOBT through laccase and electron transfer to substrate with concomitant regeneration of the nitroxide radical mediator.
Alternatively, radical mediators may function as generators of oxidative species of larger redox potential, thereby increasing the choice of substrates. An often-used mediator which utilizes this mechanism is ABTS (Scheme 2). During the initial oxidation of ABTS (E° = 690 mV) by laccase (E° = 440–790 mV), the radical cation ABTS●+ is produced. Due to the inherent instability of the radical-cation, it can spontaneously and reversibly form the dication ABTS++ (E° = 1100 mV), a strong oxidant capable of oxidizing many non-phenolic compounds [26].
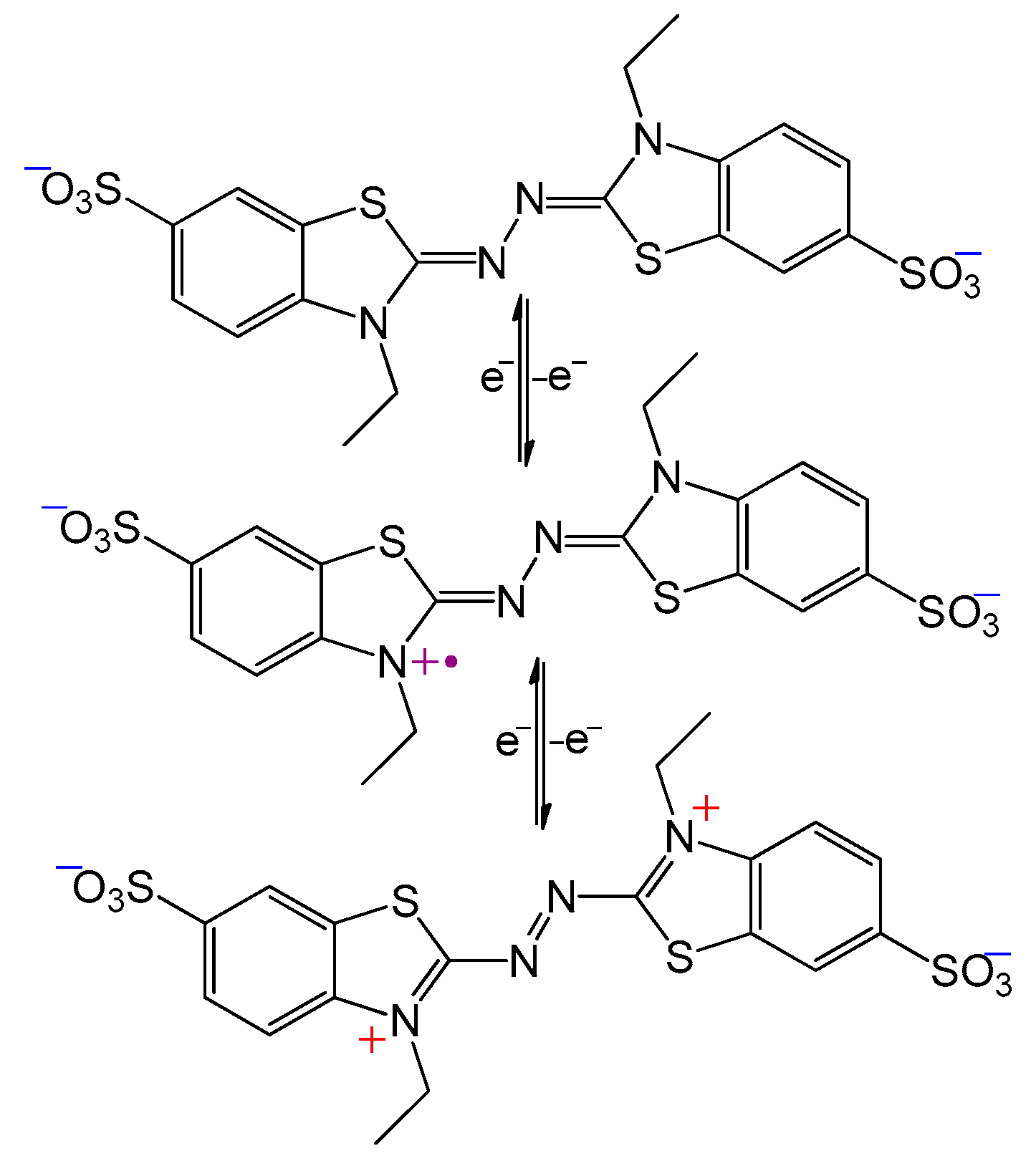
Scheme 2. Oxidation of ABTS dianion (top) to the radical cation (middle), and finally to the dication (bottom).
3. Laccase Catalysis: Commercial Applications
The success of laccases as biocatalysts in industrial applications is due primarily to their wide substrate specificity, offering great catalytic adaptability. This versatility facilitated laccase usage in the pulp and paper industry, biofuel production, food industry, manufacturing of textiles, and synthesis of small molecules, among others [27]. Mimicking their natural function in fungi, laccases are most often utilized in the pulp and paper industry as a catalyst to facilitate the degradation of lignin in paper [27][28]. The removal of lignin, which imparts a yellow color, allows for the brightening of the paper, with an identical method available for the bleaching of cotton. The use of laccase as a bleaching agent serves to replace the historically used chlorine-containing reagents, which have proven to be environmental pollutants and deleterious to ecological health. Biofuel production also utilizes laccase’s ability to degrade lignin in biomass, which would otherwise inhibit the efficient hydrolysis of polysaccharides to produce ethanol [29]. Laccase use in the food industry is predominantly involved in the oxidation and removal of undesirable phenols in beverages via radical coupling polymerization. The removal of these phenols in beverages such as wines, beers, and juices often provides stabilization, clarification, and flavor enhancement [30].
Winemaking is one area that has exploited the beneficial activity of laccase through purification through the removal of substances prone to oxidative transformation—antho-cyanins, coumaric acids, and flavans. It can also be used for process monitoring and control by biosensing, as well as in the creation of a special class of wines known as Noble Rot wines. The initial identification of oxidative enzymes (i.e., polyphenol oxidase and laccase) in the wine industry resulted from research on brown pigmentation in white grape must and juice. This reaction occurs due to oxidizing dihydroxy phenolic compounds to quinones, which, upon further oxidation, turn to brown pigments [31][32]. The first historical use of laccase focused on the removal of the phenolic content to elucidate the stabilization of wine. Using laccase isolated from Trametes versicolor, Brenna and Bianchi achieved a reduction in catechin with a model wine solution in the presence of O2 excess [33]. Browning at 420 nm increased with each successive treatment, further supporting what Macheix et al. suggested in 1991 [31]. The study also mentioned experiments carried out on Riesling grape must; however, no results were reported regarding the effectiveness of the laccase bioreactor in removing the phenolic content directly from grape must. In a more advanced study, Minussi et al. used capillary zone electrophoresis and the kinetics of phenol removal to monitor the total phenolic content, total antioxidant potential, and polyphenols to determine the efficacy in using Trametes versicolor laccase to treat grape must [34]. Phenol degradation by laccase occurred very quickly for catechins, but slowly for stilbenes and benzoic/cinnamic acid derivatives. Furthermore, in red musts, the treatment primarily showed reductions in the must antioxidant properties, while white musts showed higher reductions in the total phenols rather than total antioxidant potential. Therefore, it was concluded that laccase as a method for stabilizing wines is more feasible in white grape musts, until selectivity studies for laccase from Trametes versicolor are conducted. In a 2013 study, the laccase-catalyzed oxidation of gallic acid was compared to the chemically catalyzed procedure, and it was found that oxidative kinetics increased 3-fold in the presence of laccase [35]. Furthermore, the laccase released from Botrytis cinerea, responsible for bunch rot in wine grapes, lead to a significant decrease in the phenolic content. Though not often desirable due to its impact on wine color, there is a strong case for using laccase to reduce the total phenolic content in wines.
The initial method for measuring the polyphenol content in wine involved the use of the Folin-Ciocalteu reagent and following the reaction spectrophotometrically [36]. To improve upon this process, Gamella et al. suggested the electrochemical estimation of the polyphenol index in wine using a laccase biosensor [37]. Using Trametes versicolor laccase, they found significant differences between the laccase immobilized on a glassy carbon electrode and the Folin-Ciocalteu method. Furthermore, both methods had different specificities towards different phenolic compounds based on chemical structures. Numerous other studies, however, showed comparable results to the Folin-Ciocalteu method, including immobilized enzymes (laccase from Trametes versicolor and tyrosinase from mushroom) on graphite screen-printed electrodes modified with ferrocene [38], on a platinum-silver electrode base sensor [39], and laccase from Trametes versicolor and Trametes hirsuta using screen-printed electrodes of multi-walled carbon nanotubes as sensors [40]. In a later study, another group used laccase purified from Ganoderma sp. Rckk02 immobilized on silver/zinc oxide nanoparticles and electrochemically deposited onto a gold electrode. The oxidation potential was determined using guaiacol and a good correlation was found between the spectrophotometric method and this one. Furthermore, the biosensor was found to have retained 75% of its activity after 200 uses over 5 months [41].
Aside from improving wines through post-fermentation clarification, the fungus Botrytis cinerea can also produce a special class of wines prior to grape harvest. The production of these sweet botrytized wines is credited as originating in the Tokaj region of Hungary [42] and Schloss Johanisberg region of Germany [43], followed by the Sauternes region in France [44]. Today, these wines are also produced in regions of the United States, Australia, New Zealand, and South Africa, where favorable conditions for noble rot exist [44]. The development of desirable fungus infections, known as noble rot, occur under ideal climatic and soil conditions—notably, constant water availability and in soils poor in nutrients [45]. These conditions most readily occur due to the stimulation of infection from nocturnal humidity, morning dew, or fog that occurs in valleys or areas bordering bodies of water [46]. Grapes impacted by noble rot exhibit the withering of the fruit that is characteristic of overripening, and as such, contain a higher concentration of sugar and various aromatic hydrocarbons that constitute the unique aromas present in noble rot wines. The process by which grapes achieve these desirable attributes is due to the oxidation of aromatic metabolites through enzymatic reactions from laccase, producing new compounds such as furfural, phenylacetaldehyde, and lactones, amongst others [47].
The textile industry also benefits from the laccase-catalyzed oxidation of colorless phenols and anilines (e.g., phenylenediamines and aminophenols) for the production of a diverse range of dyes [28]. Laccases have additionally been explored as catalysts in peptide synthesis [48], the oxidation of alcohols [49], the generation of iodine from iodide [50], and the synthesis of various anti-cancer drugs [51] and antibiotics [52].
4. Laccase Use in Bioremediation Applications
The implementation of oxidoreductases for use in bioremediation applications has developed into an expansive sub discipline of biocatalysis. Laccase has specifically shown significant promise as a means of treating industrial effluents, which often contain a myriad of hazardous chemicals. Laccase has thus far demonstrated the ability to effectively oxidize a variety of compounds found in wastewaters, such as halogenated phenols [53][54] and polyaromatic hydrocarbons [25][55]. The unprecedented oxidation of fullerene C60 in water was also achieved through a laccase/linear-dendritic copolymer complex at close-to-ambient temperatures [56]. It was made possible by the activity enhancing effect of the linear-dendritic copolymer used—native laccase KM = 26.2 μmol; Vmax = 72.5 nmol·s−1·mL−1 vs. laccase–polymer complex values of 15.8 (KM) and 101.2 (Vmax), respectively [55]. This study is unique as it was able to trace for the first time a migration of potential substrates from the enveloping polymer shell to the active site. It was achieved by monitoring the fluorescence of pyrene, an environment-sensitive fluorophore that cannot be chemically transformed by the enzyme. It was established that pyrene was almost instantaneously sequestered into the hydrophobic dendritic pockets anchored at the surface of the enzyme and slowly migrated to the active site over a time span of more than 15 h (Figure 3), an important finding that was used in subsequent “green” chemistry reactions with the same polymer–enzyme complex.
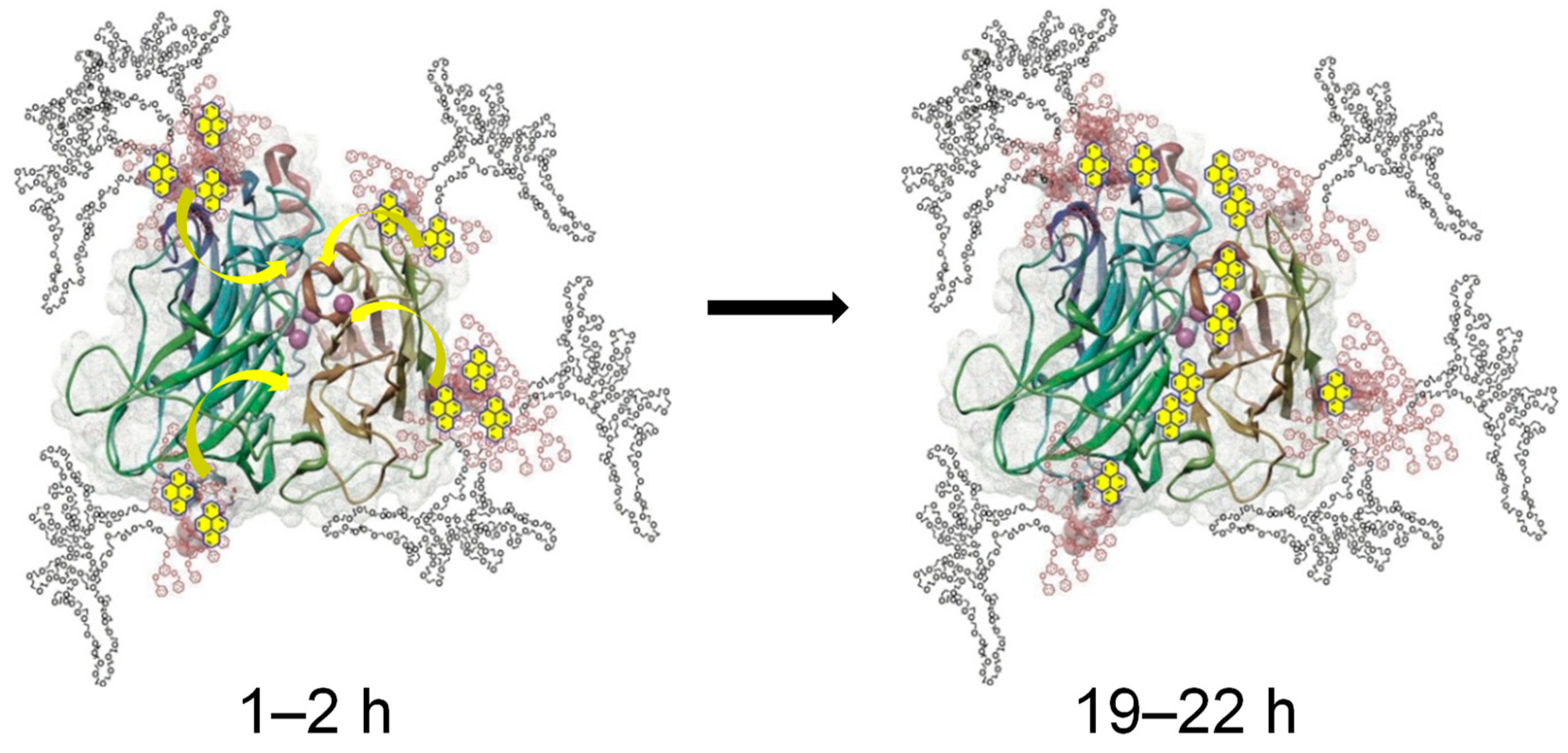
Figure 3. Substrate harvesting and migration to laccase active site, as revealed through spectrophotometric investigation of pyrene fluorescence [56].
The environmental benefits of this method compared to previously published procedures [57] were clearly distinguishable (Scheme 3); nonetheless, the editors of Angewandte Chemie, The Journal of the American Chemical Society, Journal of Molecular Catalysis B: Enzymatic, and Green Chemistry did not have the scientific courage to publish it, despite the solid experimental proof and overly positive reviews.
Laccase is also able to oxidize [58] or polymerize [59] bisphenol A and derivatives, polychlorinated biphenyls [60], dioxins [61], and other phenolic compounds [62], known as mutagens and endocrine disruptors. The removal of these hazardous chemicals by laccase occurs via radical mediated dehalogenation and/or oxidative polymerization, resulting in compounds of markedly decreased toxicity and water solubility, lowering their environmental impact. Furthermore, laccase has been validated to degrade various pharmaceuticals, with particular emphasis on steroids [63], antibiotics [64], and other micropollutants [65].
The decolorization of dyes in industrial effluents has also garnered significant attention. Due to their diverse composition, the degradation of dyes in wastewater treatment necessitates a method capable of degrading a wide range of substrates. The broad substrate specificity of laccase makes it an ideal candidate as a catalyst for the treatment of industrial effluents for the textile industry, where dyes are commonly used. Laccase has demonstrated its viability in this role by successfully oxidizing the most common classes of dyes used in the production of textiles, including azo (comprising mono-, di-, and triazo compounds) [66], triphenylmethanes [67] indigo [68], anthraquinone [69], and xanthene dyes [70]. The implementation of laccase as a catalyst in the degradation and decolorization of dyes from industrial effluents therefore serves to improve water quality by decreasing the color, turbidity, and toxicity, while most notably avoiding the production of toxic arylamines.
5. Laccase-Catalyzed Polymerizations
Mirroring many of their natural functions, laccases have also been thoroughly studied as polymerization catalysts, with many of their substrates being phenol, catechol, and aniline derivatives. An example of a phenolic compound (tyrosine) polymerization mechanism is shown in Scheme 4 [71]. Notably, this substrate is strongly hydrophobic, but the polymerization was accomplished in water facilitated by the same linear-dendritic copolymer complex.
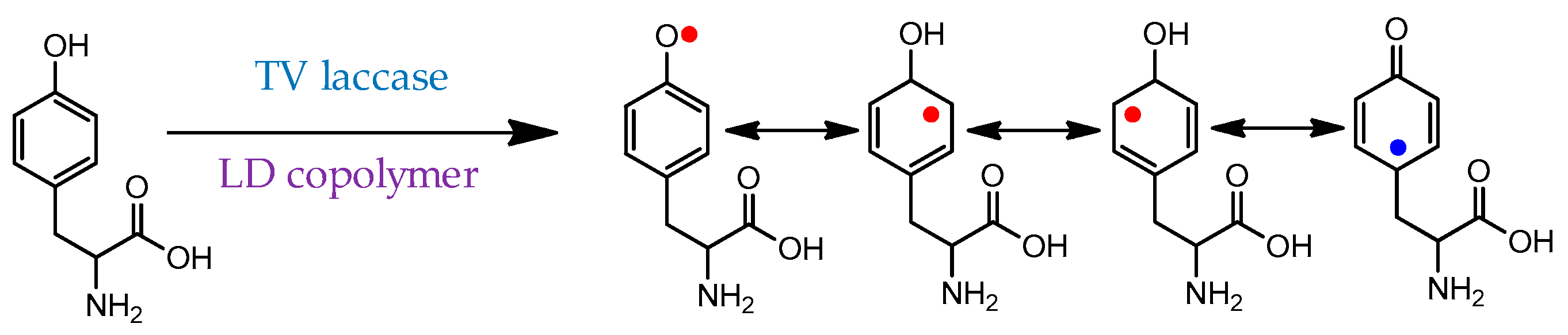
Scheme 4. Initial radical formation during the polymerization of tyrosine mediated by laccase/linear-dendritic copolymer complex [71]. Red dots—productive radicals leading to C-C or C-O couplings; blue dot—nonproductive radical.
The oxo- and ortho-centered radicals participate in sequential polyaddition reactions. The resulting polymer behaves as a typical poly-zwitterion, decreasing or increasing in size with changes in the pH of the aqueous medium [71]. This behavior hints at the flexible linear structure of the unnatural poly(tyrosine) chain, thus revealing the propagation mechanism, as shown in Scheme 5.
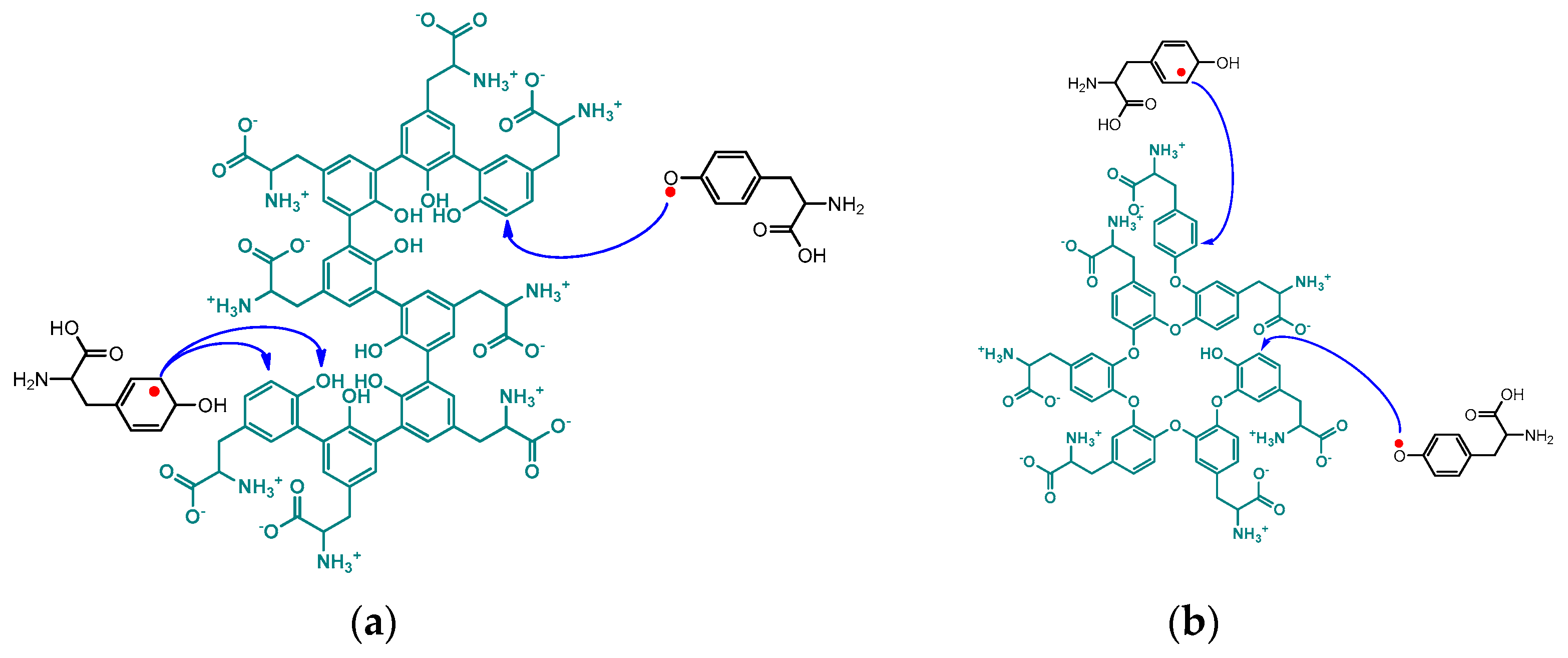
Scheme 5. Suggested propagation mechanism through addition of tyrosine monomer radicals to the chain ends of unnatural poly(tyrosine). (a) Addition to all C-C coupling chain; (b) addition to all C-O coupling chain.
A similar approach was used for the first-ever enzyme-mediated synthesis of dendronized self-assembling unnatural poly(tyrosine), poly(tyr-Gn) [72]. The number of repeating units that laccase was able to “stitch” in a polymer chain depended on the dendron generation and decreased from 12 in poly(tyr-G1) to 9 for poly(tyr-G2) and 7 in poly(tyr-G3). Nevertheless, all three polymers spontaneously aggregated in aqueous media into unique supramolecular structures.
A never-before attempted synthesis of multi-block copolymers by a single enzyme in a one-pot process was also recently accomplished. Laccase catalyzed the first “quasi-living” copolymerization through the sequential addition of multiple naturally derived phenolic comonomers conjugated with a model drug (ibuprofen) or fluorescent groups (fluorescein or rhodamine) [73]. The multi-block copolymers produced by the last method have low cytotoxicity and can undergo cellular uptake, a good indication for their promising theragnostic potential. The published protocol opens promising pathways for the construction of copolymers with properties tailor-made for specific applications
There are several reports on the laccase-mediated synthesis of poly(catechol), an electroactive polymer that is often applied as a thin film in biosensing applications [74]. Poly(phenyl ether), a commercially useful polymer due to its optical clarity and high thermal and radiation resistance, has also been produced through an oxidative radical coupling and subsequent decarboxylation of para-hydroxybenzoic acid derivatives [75].
The enzymatic synthesis of intrinsically conductive and thermally stable poly(aniline) has perhaps received the most attention. In contrast to conventional methods of poly(aniline) synthesis using horseradish peroxidase [76] or oxidizing reagents [77], the use of laccase does not require stoichiometric amounts of oxidizer or hydrogen peroxide. Laccase therefore provides a safe and inexpensive method of synthesizing poly(aniline), a polymer widely used in energy storage devices, biosensors, and anticorrosion coatings [78][79].
The use of laccase as a polymerization catalyst is not, however, limited to phenolic substrates. Reports of laccase facilitating the polymerization of vinyl monomers are numerous, with high molecular masses and yields often being achieved. Unlike the polymerization of phenolic compounds, which occurs through oxidative radical coupling, in vinyl polymerizations, laccase most often acts as a catalyst for polymerization initiation. Thus, it has been described as a catalytic initiator for the atom radical transfer polymerization of N-vinylimidazole, producing a polymer of low dispersity for use in drug delivery, fuel cell membranes, and polyionic liquids [80]. More traditional laccase-catalyzed free radical polymerizations conducted with acetylacetone as a radical mediator have used a variety of substrates, including acrylamide [81], methyl methacrylate, and styrene, for the synthesis of commercially significant polymers [82]. A limited number of laccase copolymers were formed by either vinyl comonomers [82] or phenolic and vinyl comonomers [83]. Phenolic comonomers were also grafted onto solid supports such as cellulose [84], although these have yet to attain significant commercial value.
This entry is adapted from the peer-reviewed paper 10.3390/molecules29050989
References
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase Properties, Physiological Functions, and Evolution. Int. J. Mol. Sci. 2020, 21, 966.
- Yoshida, H. Chemistry of Lacquer (Urushi). J. Chem. Soc. Trans. 1883, 43, 472–486.
- Kang, K.H.; Dec, J.; Park, H.; Bollag, J.M. Transformation of the fungicide cyprodinil by a laccase of Trametes villosa in the presence of phenolic mediators and humic acid. Water Res. 2002, 36, 4907–4915.
- Nagai, M.; Kawata, M.; Watanabe, H.; Ogawa, M.; Saito, K.; Takesawa, T. Important role of fungal intracellular laccase for melanin synthesis: Purification and characterization of an intracellular laccase from Lentinula edodes fruit bodies. Microbiology 2003, 149, 2455–2462.
- Claus, H. Laccases and their occurrences in prokaryotes. Arch. Microbiol. 2003, 179, 145–150.
- Wang, C.-Y.; Zhang, S.; Yu, Y.; Luo, Y.-C.; Liu, Q.; Ju, C.; Zhang, Y.-C.; Qu, L.-H.; Lucas, W.J.; Wang, X.; et al. MiR397b regulates both lignin content and seed number in Arabidopsis via modulating a laccase involved in lignin biosynthesis. Plant Biotchnol. J. 2014, 12, 1132–1142.
- Soares, M.P.M.; Silva-Torres, F.A.; Elias-Neto, M.; Nunes, F.M.F.; Simoes, Z.L.P.; Bitondi, M.M.G. Ecdysteroid-Dependent Expression of the Tweedle and Peroxidase Genes during Adult Cuticle Formation in the Honey Bee, Apis mellifera. PLoS ONE 2011, 6, e20513.
- Xu, F.; Berka, R.M.; Wahleithner, J.A.; Nelson, B.A.; Shuster, J.R.; Brown, S.H.; Palmer, A.E.; Solomon, E.I. Site-directed mutations in fungal laccase: Effect on redox potential, activity, and pH profile. Biochem. J. 1998, 334, 63–70.
- Xu, F.; Palmer, A.E.; Yaver, D.S.; Berka, R.M.; Gambetta, G.A.; Brown, S.H. Targeted mutations in a Trametes villosa laccase. Axial perturbations of the T1 copper. J. Biol. Chem. 1999, 274, 12372–12375.
- Freeman, J.C.; Nayar, P.G.; Begley, T.P.; Villafranca, J.J. Stoichiometry and spectroscopic identity of copper centers in phenoxazonine synthase: A new addition for the blue copper oxidase family. Biochemistry 1993, 32, 4826–4830.
- Hullo, M.F.; Moszer, I.; Danchin, A.; Martin-Verstraete, I. CotA of Bacillus substilis is a copper-dependent laccase. J. Bacteriol. 2001, 183, 5426–5430.
- Cha, J.; Cooksey, D.A. Copper resistance in Pseudomonas syringae by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. USA 1991, 88, 8915–8919.
- LaFeyette, P.R.; Erikson, K.-E.L.; Dean, J. Nucleotide sequence of a cDNA cloned encoding and acidic laccase from sycamore maple (Acer pseudoplatanus L.). Plant Physiol. 1995, 107, 667–668.
- Slomczynski, D. Biochemical Studies of Laccase from Botrytis cinerea. Masters’ Thesis, State University of New York—ESF, Syracuse, NY, USA, 1993.
- Musci, G.; Tosi, L.; Desideri, A.; Morpungo, L.; Garnier-Suillerot, A. Effects of laser radiation on Rhus vernicifera laccase, type 2 Cu-depleted laccase and stellacyanin. J. Inorg. Biochem. 1984, 20, 87–92.
- Thomas, B.R.; Yonekura, M.; Morgan, T.D.; Czapula, T.H.; Hopkins, T.L.; Kramer, K.J. A trypsin solubilized laccase from pharate pupal integument of the tobacco hornworm, Manduca sexta. Insect Biochem. 1989, 19, 611–622.
- Sugumaran, M.; Giglio, L.; Kundzicz, H.; Saul, S.; Semensi, V. Studies on the enzymes involved in puparial cuticle sclerotization in Drosophila melanogaster. Arch. Insect Biochem. Physiol. 1992, 19, 271–283.
- Sugumaran, M.; Nellaiappan, K. On the latency and nature of phenoloxidase present in the left collecterial gland of the cockroach Periplaneta americana. Arch. Insect Biochem. Physiol. 1990, 15, 165–181.
- Piontek, K.; Antorini, M.; Choinowski, T. Crystal Structure of a Laccase from the Fungus Trametes versicolor at 1.90-A Resolution Containing a Full Complement of Coppers. J. Biol. Chem. 2002, 277, 37663–37669.
- Slomczynski, D.; Nakas, J.P.; Tanenbaum, S.W. Production and Characterization of Laccase from Botrytis cinerea. Appl. Environ. Microbiol. 1995, 61, 907–912.
- Kallio, J.P.; Auer, S.; Jänis, J.; Andberg, M.; Kruus, K.; Rouvinen, J.; Koivula, A.; Hakulinen, N. Structure–Function Studies of a Melanocarpus albomyces Laccase Suggest a Pathway for Oxidation of Phenolic Compounds. J. Mol. Biol. 2009, 392, 895–909.
- Baldrian, P. Fungal laccases—Occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242.
- Jones, S.; Solomon, E.I. Electron transfer and reaction mechanism of laccases. Cell. Mol. Life Sci. 2015, 72, 869–883.
- Cañas, A.I.; Camarero, S. Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010, 28, 694–705.
- Gitsov, I.; Lambrych, K.; Lu, P.; Nakas, J.; Ryan, J.; Tanenbaum, S. Nondestructive Regioselective Modification of Laccase by Linear-Dendritic Copolymers. Enhanced Oxidation of Benzo-α-Pyrene in Water. In Polymer Biocatalysis and Biomaterials; Cheng, H.N., Gross, R.A., Eds.; American Chemical Society: Washington, DC, USA, 2005; ACS Symp. Ser.; Volume 900, pp. 80–94.
- Costa, I.O.; Morais, J.R.F.; Dantas, J.M.M.; Gonçalves, L.R.B.; Santos, E.S.; Rios, N.S. Enzyme immobilization technology as a tool to innovate in the production of biofuels: A special review of the Cross-Linked Enzyme Aggregates (CLEAs) strategy. Enzyme Microb Technol. 2023, 170, 110300.
- Cannatelli, M.D.; Ragauskas, A.J. Two Decades of Laccases: Advancing Sustainability in the Chemical Industry. Chem. Rec. 2017, 17, 122–140.
- Mate, D.M.; Alcalde, M. Laccase: A multipurpose biocatalysts at the forefront of biotechnology. Microb. Biotechnol. 2017, 10, 1457–1467.
- Curran, L.M.C.L.K.; Pham, L.T.M.; Sale, K.L.; Simmons, B.A. Review of advances in the development of laccases for the valorization of lignin to enable the production of lignocellulosic biofuels and bioproducts. Biotechnol. Adv. 2022, 54, 107809.
- Minussi, R.C.; Pastore, G.M.; Durán, N. Potential applications of laccase in the food industry. Trends Food Sci. Technol. 2002, 13, 205–216.
- Macheix, J.J.; Fleuriet, A.; Sapis, J.C.; Lee, C.Y. Phenolic compounds and polyphenoloxidase in relation to browning in grapes and wines. Crit. Rev. Food Sci. Nutr. 1991, 30, 441–486.
- Li, H.; Guo, A.; Wang, H. Mechanisms of oxidative browning of wine. Food Chem. 2008, 108, 1–13.
- Brenna, O.; Bianchi, E. Immobilised laccase for phenolic removal in must and wine. Biotechnol. Lett. 1994, 16, 35–40.
- Minussi, R.C.; Rossi, M.; Bologna, L.; Rotilio, D.; Pastore, G.M.; Durán, N. Phenols removal in musts: Strategy for wine stabilization by laccase. J. Mol. Catal. B Enzym. 2007, 45, 102–107.
- Zinnai, A.; Venturi, F.; Sanmartin, C.; Quartacci, M.; Andrich, G. Chemical and Laccase catalysed oxidation of gallic acid: Determination of kinetic parameters. Res. J. Biotechnol. 2013, 8, 62–65.
- Valaer, P. Methods of Analysis of Wine. J. Assoc. Off. Agric. Chem. 1947, 30, 327–331.
- Gamella, M.; Campuzano, S.; Reviejo, A.J.; Pingarrón, J.M. Electrochemical estimation of the polyphenol index in wines using a laccase biosensor. J. Agric. Food Chem. 2006, 54, 7960–7967.
- Montereali, M.R.; Seta, L.D.; Vastarella, W.; Pilloton, R. A disposable Laccase-Tyrosinase based biosensor for amperometric detection of phenolic compounds in must and wine. J. Mol. Catal. B Enzym. 2010, 64, 189–194.
- Gil, D.M.A.; Rebelo, M.J.F. Evaluating the antioxidant capacity of wines: A laccase-based biosensor approach. Eur. Food Res. Technol. 2010, 231, 303–308.
- Di Fusco, M.; Tortolini, C.; Deriu, D.; Mazzei, F. Laccase-based biosensor for the determination of polyphenol index in wine. Talanta 2010, 81, 235–240.
- Chawla, S.; Rawal, R.; Kumar, D.; Pundir, C.S. Amperometric determination of total phenolic content in wine by laccase immobilized onto silver nanoparticles/zinc oxide nanoparticles modified gold electrode. Anal. Biochem. 2012, 430, 16–23.
- Magyar, I. Botrytized Wines. Adv. Food Nutr. Res. 2011, 63, 147–206.
- Thudichum, J.L.W.; Dupré, A. A Treatise on the Origin, Nature, and Varieties of Wine: Being a Complete Manual of Viticulture and Œnology; Macmillan and Co.: London, UK; New York, NY, USA, 1872; p. 760.
- Jackson, R.S. Specific and Distinctive Wine Styles in Wine Science: Principles and Applications; Academic Press: London, UK, 2008; pp. 520–576.
- Modesti, M.; Alfieri, G.; Chieffo, C.; Mencarelli, F.; Vannini, A.; Catalani, A.; Chilosi, G.; Bellincontro, A. Destructive and non-destructive early detection of postharvest noble rot (Botrytis cinerea) in wine grapes aimed at producing high-quality wines. J. Sci. Food Agric. 2023; early view.
- Magyar, I.; Soos, J. Botrytized wines—Current perspectives. Int. J. Wine Res. 2016, 8, 29–39.
- Bailly, S.; Jerkovic, V.; Marchand-Brynaert, J.; Collin, S. Aroma extraction dilution analysis of sauternes wines. Key role of polyfunctional thiols. J. Agric. Food Chem. 2006, 54, 7227–7234.
- Semenov, A.N.; Lomonosova, I.V.; Berezin, V.I.; Titov, M.I. Peroxidase and laccase as catalysts for removal of the phenylhydrazide protecting group under mild conditions. Biotechnol. Bioeng. 1993, 42, 1137–1141.
- Liu, J.; Wu, S.; Li, Z. Recent advances in enzymatic oxidation of alcohols. Curr. Opin. Chem. Biol. 2018, 43, 77–86.
- Xu, F. Catalysis of novel enzymatic iodide oxidation by fungal laccase. Appl. Biochem. Biotechnol. 1996, 59, 221–230.
- Chauhan, P.S.; Kumarasamy, M.; Sosnik, A.; Danino, D. Enhanced Thermostability and Anticancer Activity in Breast Cancer Cells of Laccase Immobilized on Pluronic-Stabilized Nanoparticles. ACS Appl. Mater. Interfaces 2019, 11, 39436–39448.
- Mikolasch, A.; Hildebrandt, O.; Schlüter, R.; Hammer, E.; Witt, S.; Lindequist, U. Targeted synthesis of novel β-lactam antibiotics by laccase-catalyzed reaction of aromatic substrates selected by pre-testing for their antimicrobial and cytotoxic activity. Appl. Microbiol. Biotechnol. 2016, 100, 4885–4899.
- Uhnáková, B.; Petrícková, A.; Biedermann, D.; Homolka, L.; Vejvoda, V.; Bednár, P.; Papousková, B.; Sulc, M.; Martínková, L. Biodegradation of brominated aromatics by cultures and laccase of Trametes versicolor. Chemosphere 2009, 76, 826–832.
- Zhang, J.; Liu, X.; Xu, Z.; Chen, H.; Yang, Y. Degradation of chlorophenols catalyzed by laccase. Int. Biodeter. Biodegr. 2008, 61, 351–356.
- Gitsov, I.; Lambrych, K.R.; Nakas, J.; Lu, P.; Ryan, J.; Omori, S.; Tanenbaum, S.W. Nondestructive Regioselective Modification of Laccase by Linear-Dendritic Copolymers. Enhanced Oxidation of Polyaromatic Hydrocarbons in Water. Polym. Prepr. 2003, 44, 143–144.
- Gitsov, I.; Simonyan, A.; Wang, L.; Krastanov, A.; Tanenbaum, S.W.; Kiemle, D. Polymer-assisted biocatalysis: Unprecedented enzymatic oxidation of fullerene in aqueous medium. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 119–126.
- Chiang, L.Y.; Upasani, R.B.; Swirczewski, J.W.; Soled, S. Evidence of hemiketals incorporated in the structure of fullerols derived from aqueous acid chemistry. J. Am. Chem. Soc. 1993, 115, 5453–5457.
- Lin, H.; Yu, Z.J.; Wang, Q.; Liu, Y.J.; Jiang, L.; Xu, C.; Xian, M. Application of Laccase Catalysis in Bond Formation and Breakage: A Review. Catalysts 2023, 13, 750.
- Gitsov, I.; Simonyan, A. “Green” Synthesis of Bisphenol Polymers and Copolymers, Mediated by Supramolecular Complexes of Laccase and Linear-Dendritic Block Copolymers. In Green Polymer Chemistry: Biocatalysis and Materials II; Cheng, H.N., Gross, R.A., Eds.; American Chemical Society: Washington, DC, USA, 2013; ACS Symposium Series; Volume 1144, pp. 121–139.
- Novotný, Č.; Vyas, B.R.M.; Erbanová, P.; Kubátová, A.; Šašek, A. Removal of PCBs by various white rot fungi in liquid cultures. Folia Microbiol. 1997, 42, 136–142.
- Mori, T.; Kondo, R. Oxidation of chlorinated dibenzo-p-dioxin and dibenzofuran by white-rot fungus, Phlebia lindtneri. FEMS Microbiol. Lett. 2002, 216, 223–227.
- Dencheva, N.; Oliveira, S.; Braz, J.; Getya, D.; Malfois, M.; Denchev, Z.; Gitsov, I. Magnetically responsive PA6 microparticles with immobilized laccase show high catalytic efficiency in the enzymatic treatment of catechol. Catalysts 2021, 11, 239.
- Gitsov, I.; Simonyan, A.; Krastanov, A.; Tanenbaum, S. Green oxidation of steroids in nano-reactors assembled from laccase and linear-dendritic copolymers. In Polymer Biocatalysis and Biomaterials II; Cheng, H.N., Gross, R.A., Eds.; American Chemical Society: Washington, DC, USA, 2008; ACS Symposium Series; Volume 999, pp. 110–128.
- Yang, J.; Li, W.; Ng, T.B.; Deng, X.; Lin, J.; Ye, X. Laccases: Production, Expression Regulation and Application in Pharmaceutical Biodegradation. Front. Microbiol. 2017, 8, 832.
- Janusz, G.; Skwarek, E.; Pawlik, A. Potential of Laccase as a Tool for Biodegradation of Wastewater Micropollutants. Water 2023, 15, 3770.
- Kanagaraj, J.; Senthilvelan, T.; Panda, R. Degradation of azo dyes by laccase: Biological method to reduce pollution load in dye wastewater. Clean Technol. Environ. Policy 2015, 17, 1443–1456.
- Chhabra, M.; Mishra, S.; Sreekrishnan, T.R. Laccase/mediator assisted degradation of triarylmethane dyes in a continuous membrane reactor. J. Biotechnol. 2009, 143, 69–78.
- Campos, R.; Kandelbauer, A.; Robra, K.H.; Cavaco-Paulo, A.; Gubitz, G.M. Indigo degradation with purified laccases from Trametes hirsuta and Sclerotium rolfsii. J. Biotechnol. 2001, 89, 131–139.
- Soares, G.M.; Costa-Ferreira, M.; Pessoa, M.T. Decolorization of an anthraquinone-type dye using a laccase formulation. Bioresour. Technol. 2001, 79, 171–177.
- Itoh, K.; Yatome, C. Decolorization and Degradation of Xanthene Dyes by a White Rot Fungus, Coriolus Versicolor. J. Environ. Sci. Health Part A Tox. Haz. Subst. Environ. Eng. 2011, 39, 2382–2389.
- Gitsov, I.; Wang, L.; Vladimirov, N.; Simonyan, A.; Kiemle, D.J.; Schutz, A. “Green” Synthesis of Unnatural Poly (Amino Acid)s with Zwitterionic Character and pH-Responsive Solution Behavior, Mediated by Linear–Dendritic Laccase Complexes. Biomacromolecules 2014, 15, 4082–4095.
- Liu, X.; Wang, L.; Gitsov, I. Novel Amphiphilic Dendronized Copolymers Formed by Enzyme-Mediated “Green” Polymerization. Biomacromolecules 2021, 22, 1706–1720.
- Scheibel, D.M.; Guo, D.; Luo, J.; Gitsov, I. A single enzyme mediates the “quasi-living” formation of multiblock copolymers with a broad biomedical potential. Biomacromolecules 2020, 21, 2132–2146.
- Aktas, N.; Tanyolac, A. Reaction conditions for laccase catalyzed polymerization of catechol. Bioresour. Technol. 2003, 87, 209–214.
- Marjasvaara, A.; Torvinen, M.; Kinnunen, H.; Vainiotalo, P. Laccase-Catalyzed Polymerization of Two Phenolic Compounds Studied by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight and Electrospray Ionization Fourier Transform Ion Cyclotron Resonance Mass Spectrometry with Collision-Induced Dissociation Experiments. Biomacromolecules 2006, 7, 1604–1609.
- Alvarez, S.; Manolache, S.; Denes, F. Synthesis of polyaniline using horseradish peroxidase immobilized on plasma-functionalized polyethylene surfaces as initiator. J. Appl. Polym. Sci. 2003, 88, 369–379.
- Gospodinova, N.; Terlemezyan, L. Conducting polymers prepared by oxidative polymerization: Polyaniline. Progr. Polym. Sci. 1998, 23, 1443–1484.
- Karamyshev, A.V.; Shleev, S.V.; Koroleva, O.V.; Yaropolov, A.I.; Sakharov, I.Y. Laccase-catalyzed synthesis of conducting polyaniline. Enzyme Microb. Technol. 2003, 33, 556–564.
- Walde, P.; Kashima, K.; Ćirić-Marjanović, G. Synthesizing Polyaniline with Laccase/O2 as Catalyst. Front. Bioeng. Biotechnol. 2019, 77, 165.
- Fodor, C.; Gajewska, B.; Rifaie-Graham, O.; Apedende, E.A.; Pollard, J.; Bruns, N. Laccase-catalyzed controlled radical polymerization of N-vinylimidazole. Polym. Chem. 2016, 7, 6617–6625.
- Ikeda, R.; Taraka, H.; Uyama, H.; Kobayashi, S. Laccase-catalyzed polymerization of acrylamide. Macromol. Rapid Commun. 1998, 19, 423–425.
- Tsujimoto, T.; Uyama, H.; Kobayashi, S. Polymerization of Vinyl Monomers Using Oxidase Catalysis. Macromol. Biosci. 2001, 1, 228–232.
- Mai, C.; Schormann, W.; Huttermann, A. Chemo-enzymatically induced copolymerization of phenolics with acrylate compounds. Appl. Microbiol. Biotechnol. 2001, 55, 177–186.
- Elegir, G.; Kindl, A.; Sadocco, P.; Orlandi, M. Development of antimicrobial cellulose packaging through laccase-mediated grafting of phenolic compounds. Enzym. Microb. Technol. 2008, 43, 84–92.
This entry is offline, you can click here to edit this entry!
