Although alcohol is one of the most important etiologic agents in the development of chronic liver disease worldwide, also recognized as a promoter of carcinogenesis, several studies have shown a beneficial effect of moderate consumption in terms of reduced cardiovascular morbidity and mortality. Whether this benefit is also present in patients with liver disease due to other causes (viral, metabolic, and others) is still debated. Although there is no clear evidence emerging from guidelines and scientific literature, total abstention from drinking is usually prescribed in clinical practice.
- alcohol consumption
- chronic liver disease
- NAFLD
- viral hepatitis
- HBV
- HCV
1. Introduction
2. Drinking Quantification
3. NAFLD
|
Reference |
Population Sample |
Modest Alcohol Use Definition |
Main Results |
|---|---|---|---|
|
Chang et al. 2019 [27] |
58,927 |
<30 g/d in men <20 g/d in women |
progression of fibrosis |
|
Kawamura et al. 2016 [28] |
9959 |
<40 g/d |
increased risk of HCC development in modest alcohol users |
|
Moriya et al. 2015 [29] |
5297 |
<280 g/w |
protective effect of alcohol use against steatosis in men (in women only if <70 g/w) |
|
Åberg et al. 2019 [30] |
8345 |
<30 g/d in men <20 g/d in women |
increased liver events if intake is above 9 g/d; reduced CV mortality in drinkers (up to 49 g/d) |
|
Hajifathalian et al. 2019 [31] |
4568 |
<1.5 drink/d |
reduced risk of death in moderate consumers |
|
Younossi et al. 2019 [32] |
4264 |
>3 drinks/w <2 drinks/d for men |
only excessive alcohol consumption increases death rate |
|
Akahane et al. 2020 [33] |
2429 |
<60 g/d |
alcohol use is inversely associated with NAFLD |
|
Patel et al. 2017 [34] |
151 |
<20 g/d |
no protective effect of moderate alcohol use |
4. Chronic Viral Hepatitis
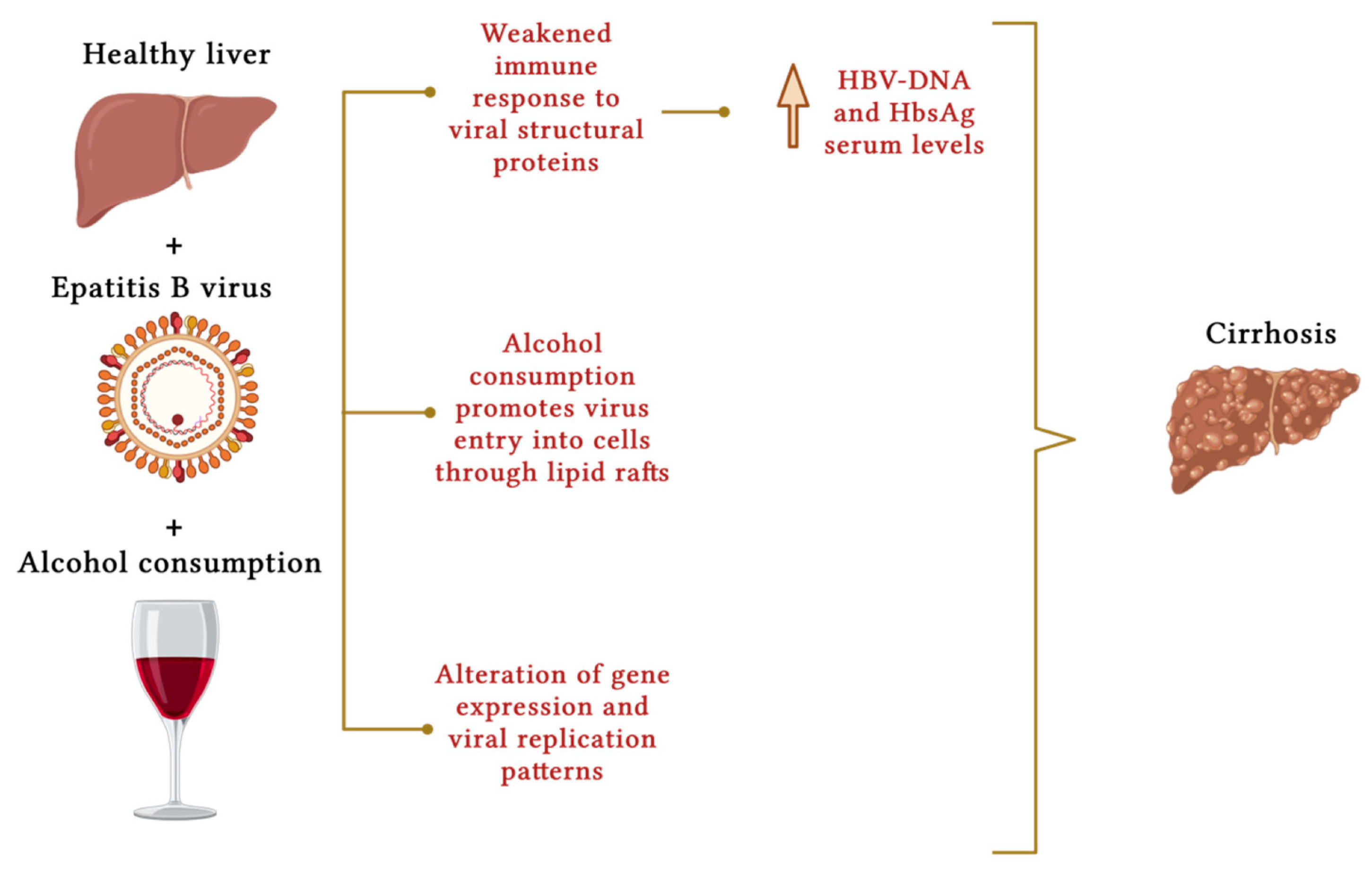
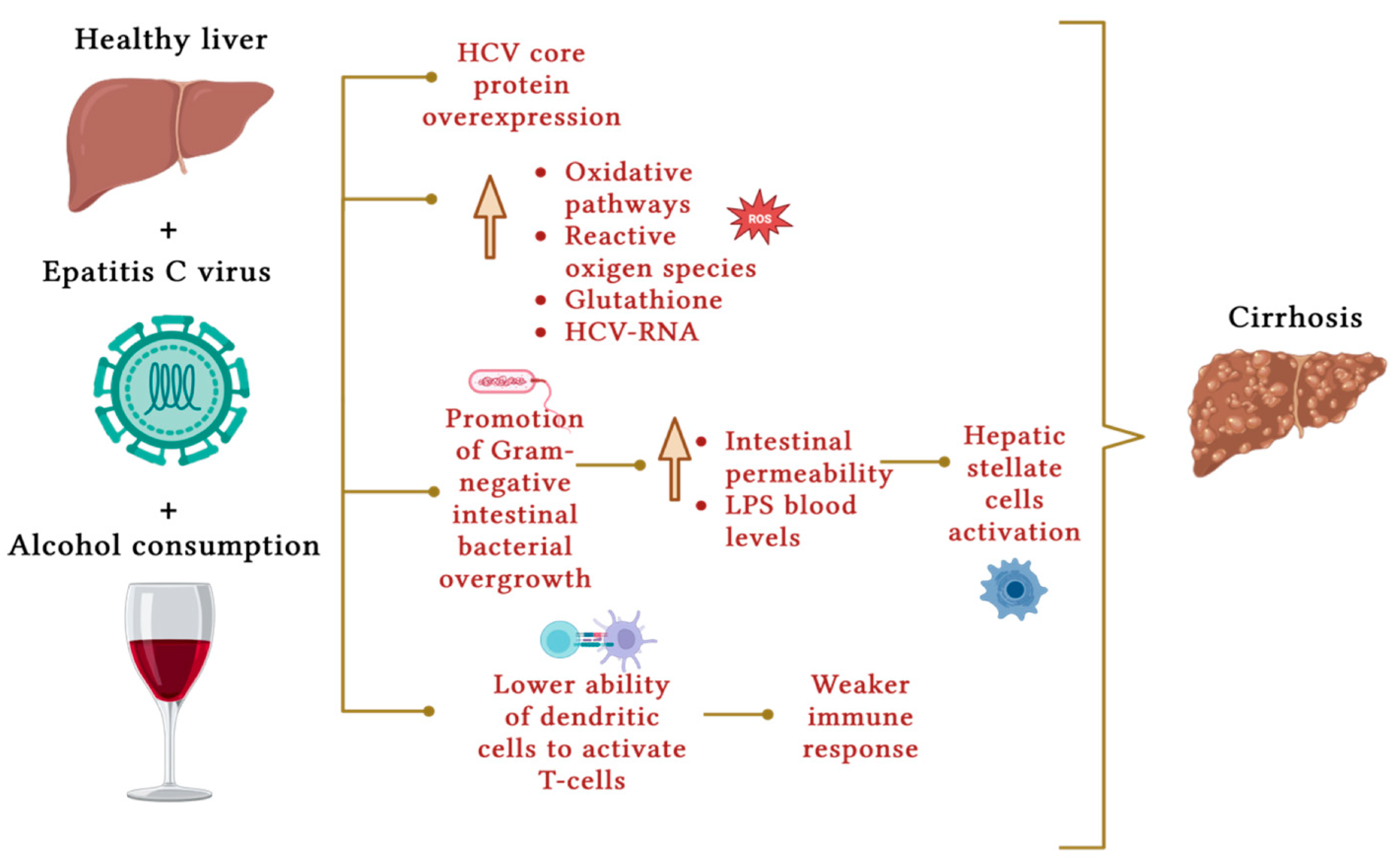
5. Hemochromatosis
This entry is adapted from the peer-reviewed paper 10.3390/nu16050613
References
- WHO Global Status Report on Noncommunicable Diseases 2014; WHO Library Cataloguing-in-Publication Data; WHO: Geneva, Switzerland, 2014.
- Esser, M.B.; Leung, G.; Sherk, A.; Bohm, M.K.; Liu, Y.; Lu, H.; Naimi, T.S. Estimated Deaths Attributable to Excessive Alcohol Use among US Adults Aged 20 to 64 Years, 2015 to 2019. JAMA Netw. Open 2022, 5, e2239485.
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233.
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J.Cancer 2015, 112, 580–593.
- Rumgay, H.; Murphy, N.; Ferrari, P.; Soerjomataram, I. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients 2021, 13, 3173.
- Rumgay, H.; Shield, K.; Charvat, H.; Ferrari, P.; Sornpaisarn, B.; Obot, I.; Islami, F.; Lemmens, V.E.P.P.; Rehm, J.; Soerjomataram, I. Global burden of cancer in 2020 attributable to alcohol consumption: A population-based study. Lancet Oncol. 2021, 22, 1071–1080.
- Van de Luitgaarden, I.A.T.; Bardach, A.E.; Espinola, N.; Schrieks, I.C.; Grobbee, D.E.; Beulens, J.W.J. Alcohol-attributable burden of cancer in Argentina. BMC Public Health 2022, 22, 124.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of alcohol-related liver disease. J. Hepatol. 2018, 69, 154–181.
- Corrao, G.; Rubbiati, L.; Bagnardi, V.; Zambon, A.; Poikolainen, K. Alcohol and coronary heart disease: A meta-analysis. Addiction 2000, 95, 1505–1523.
- Klatsky, A.L. Alcohol and cardiovascular diseases: Where do we stand today? J. Intern. Med. 2015, 278, 238–250.
- Roerecke, M. Alcohol’s Impact on the Cardiovascular System. Nutrients 2021, 13, 3419.
- Minzer, S.; Losno, R.A.; Casas, R. The Effect of Alcohol on Cardiovascular Risk Factors: Is There New Information? Nutrients 2020, 12, 912.
- Rehm, J.; Roerecke, M. Cardiovascular effects of alcohol consumption. Trends Cardiovasc. Med. 2017, 27, 534–538.
- WHO Food Based Dietary Guidelines; WHO: Geneva, Switzerland, 2003.
- Saunders, J.B.; Aasland, O.G.; Babor, T.F.; de la Fuente, J.R.; Grant, M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction 1993, 88, 791–804.
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285.
- Younossi, Z.M.; Stepanova, M.; Afendy, M.; Fang, Y.; Younossi, Y.; Mir, H.; Srishord, M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin. Gastroenterol. Hepatol. 2011, 9, 524–530.
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84.
- Riazi, K.; Azhari, H.; Charette, J.H.; Underwood, F.E.; King, J.A.; Afshar, E.E.; Swain, M.G.; Congly, S.E.; Kaplan, G.G.; Shaheen, A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861.
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 11–20.
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression from NAFLD to NASH. Transplantation 2019, 103, e1–e13.
- Schuppan, D.; Surabattula, R.; Wang, X.Y. Determinants of fibrosis progression and regression in NASH. J. Hepatol. 2018, 68, 238–250.
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402.
- Targher, G.; Byrne, C.D.; Lonardo, A.; Zoppini, G.; Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: A meta-analysis. J. Hepatol. 2016, 65, 589–600.
- Hrelia, S.; Di Renzo, L.; Bavaresco, L.; Bernardi, E.; Malaguti, M.; Giacosa, A. Moderate Wine Consumption and Health: A Narrative Review. Nutrients 2022, 15, 175.
- Sookoian, S.; Castaño, G.O.; Pirola, C.J. Modest alcohol consumption decreases the risk of non-alcoholic fatty liver disease: A meta-analysis of 43,175 individuals. Gut 2014, 63, 530–532.
- Chang, Y.; Cho, Y.K.; Kim, Y.; Sung, E.; Ahn, J.; Jung, H.S.; Yun, K.E.; Shin, H.; Ryu, S. Nonheavy Drinking and Worsening of Noninvasive Fibrosis Markers in Nonalcoholic Fatty Liver Disease: A Cohort Study. Hepatology 2019, 69, 64–75.
- Kawamura, Y.; Arase, Y.; Ikeda, K.; Akuta, N.; Kobayashi, M.; Saitoh, S.; Suzuki, F.; Suzuki, Y.; Inao, M.; Mochida, S.; et al. Effects of Alcohol Consumption on Hepatocarcinogenesis in Japanese Patients with Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2016, 14, 597–605.
- Moriya, A.; Iwasaki, Y.; Ohguchi, S.; Kayashima, E.; Mitsumune, T.; Taniguchi, H.; Ando, M.; Yamamoto, K. Roles of alcohol consumption in fatty liver: A longitudinal study. J. Hepatol. 2015, 62, 921–927.
- Åberg, F.; Puukka, P.; Salomaa, V.; Männistö, S.; Lundqvist, A.; Valsta, L.; Perola, M.; Färkkilä, M.; Jula, A. Risks of Light and Moderate Alcohol Use in Fatty Liver Disease: Follow-Up of Population Cohorts. Hepatology 2020, 71, 835–848.
- Hajifathalian, K.; Torabi Sagvand, B.; McCullough, A.J. Effect of Alcohol Consumption on Survival in Nonalcoholic Fatty Liver Disease: A National Prospective Cohort Study. Hepatology 2019, 70, 511–521.
- Younossi, Z.M.; Stepanova, M.; Ong, J.; Yilmaz, Y.; Duseja, A.; Eguchi, Y.; El Kassas, M.; Castellanos-Fernandez, M.; George, J.; Jacobson, I.M.; et al. Effects of Alcohol Consumption and Metabolic Syndrome on Mortality in Patients with Nonalcoholic and Alcohol-Related Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2019, 17, 1625–1633.
- Akahane, T.; Namisaki, T.; Kaji, K.; Moriya, K.; Kawaratani, H.; Takaya, H.; Sawada, Y.; Shimozato, N.; Fujinaga, Y.; Furukawa, M.; et al. Chronic Alcohol Consumption is Inversely Associated with Insulin Resistance and Fatty Liver in Japanese Males. Nutrients 2020, 12, 1036.
- Patel, P.J.; Smith, D.; Connor, J.P.; Horsfall, L.U.; Hayward, K.L.; Hossain, F.; Williams, S.; Johnson, T.; Stuart, K.A.; Brown, N.N.; et al. Alcohol Consumption in Diabetic Patients with Nonalcoholic Fatty Liver Disease. Can. J. Gastroenterol. Hepatol. 2017, 2017, 7927685.
- Schweitzer, A.; Horn, J.; Mikolajczyk, R.T.; Krause, G.; Ott, J.J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: A systematic review of data published between 1965 and 2013. Lancet 2015, 386, 1546–1555.
- World Health Organization. 2017. Global Hepatitis Report. Available online: https://www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ (accessed on 27 May 2023).
- Kawagishi, N.; Suda, G.; Onozawa, M.; Kimura, M.; Maehara, O.; Ohara, M.; Izumi, T.; Umemura, M.; Ito, J.; Nakai, M.; et al. Comparing the risk of hepatitis B virus reactivation between direct-acting antiviral therapies and interferon-based therapies for hepatitis C. J. Viral Hepat. 2017, 24, 1098–1106.
- Conde, I.; Vinaixa, C.; Berenguer, M. Hepatitis C-related cirrhosis. Current status. Cirrosis por hepatitis C. Estado actual. Med. Clin. 2017, 148, 78–85.
- Ahmad, J.; Eng, F.J.; Branch, A.D. HCV and HCC: Clinical update and a review of HCC-associated viral mutations in the core gene. Semin. Liver Dis. 2011, 31, 347–355.
- Singal, A.K.; Anand, B.S. Mechanisms of synergy between alcohol and hepatitis C virus. Clin. Gastroenterol. 2007, 41, 761–772.
- Jamal, M.M.; Saadi, Z.; Morgan, T.R. Alcohol and hepatitis C. Digit Dis. 2005, 23, 285–296.
- Tang, L.S.Y.; Covert, E.; Wilson, E.; Kottilil, S. Chronic Hepatitis B Infection: A Review. JAMA 2018, 319, 1802–1813.
- Yuan, J.M.; Govindarajan, S.; Arakawa, K.; Yu, M.C. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer 2004, 101, 1009–1017.
- Kubo, S.; Kinoshita, H.; Hirohashi, K.; Tanaka, H.; Tsukamoto, T.; Shuto, T.; Kuroki, T. High malignancy of hepatocellular carcinoma in alcoholic patients with hepatitis C virus. Surgery 1997, 121, 425–429.
- Ganesan, M.; Eikenberry, A.; Poluektova, L.Y.; Kharbanda, K.K.; Osna, N.A. Role of alcohol in pathogenesis of hepatitis B virus infection. World J. Gastroenterol. 2020, 26, 883–903.
- Guo, G.H.; Tan, D.M.; Zhu, P.A.; Liu, F. Hepatitis B virus X protein promotes proliferation and upregulates TGF-beta1 and CTGF in human hepatic stellate cell line, LX-2. Hepatobiliary Pancreat. Dis. Int. 2009, 8, 59–64.
- Iida-Ueno, A.; Enomoto, M.; Tamori, A.; Kawada, N. Hepatitis B virus infection and alcohol consumption. World J. Gastroenterol. 2017, 23, 2651–2659.
- Geissler, M.; Gesien, A.; Wands, J.R. Chronic ethanol effects on cellular immune responses to hepatitis B virus envelope protein: An immunologic mechanism for induction of persistent viral infection in alcoholics. Hepatology 1997, 26, 764–770.
- Dolganiuc, A. Alcohol and Viral Hepatitis: Role of Lipid Rafts. Alcohol. Res. 2015, 37, 299–309.
- Larkin, J.; Clayton, M.M.; Liu, J.; Feitelson, M.A. Chronic ethanol consumption stimulates hepatitis B virus gene expression and replication in transgenic mice. Hepatology 2001, 34, 792–797.
- Testino, G.; Leone, S.; Borro, P. Alcohol and hepatocellular carcinoma: A review and a point of view. World J. Gastroenterol. 2014, 20, 15943–15954.
- Lévy, L.; Renard, C.A.; Wei, Y.; Buendia, M.A. Genetic alterations and oncogenic pathways in hepatocellular carcinoma. Ann. N. Y. Acad. Sci. 2002, 963, 21–36.
- Otani, K.; Korenaga, M.; Beard, M.R.; Li, K.; Qian, T.; Showalter, L.A.; Singh, A.K.; Wang, T.; Weinman, S.A. Hepatitis C virus core protein, cytochrome P450 2E1, and alcohol produce combined mitochondrial injury and cytotoxicity in hepatoma cells. Gastroenterology 2005, 128, 96–107.
- Swietek, K.; Juszczyk, J. Reduced glutathione concentration in erythrocytes of patients with acute and chronic viral hepatitis. J. Viral Hepat. 1997, 4, 139–141.
- Szabo, G. Gut-liver axis in alcoholic liver disease. Gastroenterology 2015, 148, 30–36.
- Mandrekar, P.; Szabo, G. Signalling pathways in alcohol-induced liver inflammation. J. Hepatol. 2009, 50, 1258–1266.
- Bain, C.; Fatmi, A.; Zoulim, F.; Zarski, J.P.; Trépo, C.; Inchauspé, G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 2001, 120, 512–524.
- Rigamonti, C.; Mottaran, E.; Reale, E.; Rolla, R.; Cipriani, V.; Capelli, F.; Boldorini, R.; Vidali, M.; Sartori, M.; Albano, E. Moderate alcohol consumption increases oxidative stress in patients with chronic hepatitis C. Hepatology 2003, 38, 42–49.
- Cromie, S.L.; Jenkins, P.J.; Bowden, D.S.; Dudley, F.J. Chronic hepatitis C: Effect of alcohol on hepatitic activity and viral titre. J. Hepatol. 1996, 25, 821–826.
- Niemelä, O.; Parkkila, S.; Britton, R.S.; Brunt, E.; Janney, C.; Bacon, B. Hepatic lipid peroxidation in hereditary hemochromatosis and alcoholic liver injury. J. Lab. Clin. Med. 1999, 133, 451–460.
- Powell, L.W. Normal human iron storage and its relation to ethanol consumption. Australas. Ann. Med. 1966, 15, 110–115.
- Fletcher, L.M.; Powell, L.W. Hemochromatosis and alcoholic liver disease. Alcohol 2003, 30, 131–136.
- Beutelspacher, S.C.; Serbecic, N.; Tan, P.H.; Mehrabi, M.; Nielsen, P.; Yamane, Y. Low dose-ethanol modulates toxic effect of iron-overloading in the liver. J. Nutr. Sci. Vitaminol. 2004, 50, 78–86.
