Aptamers developed using in vitro Systematic Evolution of Ligands by Exponential Enrichment (SELEX) technology are single-stranded nucleic acids 10–100 nucleotides in length. Their targets, often with specificity and high affinity, range from ions and small molecules to proteins and other biological molecules as well as larger systems, including cells, tissues, and animals. Aptamers often rival conventional antibodies with improved performance, due to aptamers’ unique biophysical and biochemical properties, including small size, synthetic accessibility, facile modification, low production cost, and low immunogenicity. Therefore, there is sustained interest in engineering and adapting aptamers for many applications, including diagnostics and therapeutics.
1. Introduction
Aptamers are single-stranded (ss) oligonucleotides (RNA or DNA) that bind to their respective targets with high selectivity and affinity due to their unique three-dimensional structures. Generally, aptamers are chosen using Systematic Evolution of Ligands by Exponential Enrichment (SELEX), first used in 1990 [
1,
2]. Since then, fundamental advances have led to chemically engineered aptamers with higher binding affinities in more efficient and versatile workflows. Examples of advances include Slow Off-Rate Modified Aptamers (SOMAmer) [
3,
4], aptamers generated from expanded DNA “alphabets” [
5,
6], the adaptable amplification of synthetic oligonucleotides (evolution of sequence-defined aptamers) [
7], and the high-throughput automation of the SELEX process [
8].
Chemically engineered aptamers are envisioned as a family of potential artificial ligands for biomarker and drug discovery, diagnostics, drug delivery, and other therapeutic applications. Aptamers may also be used in process chemistry, for example, as chromatographic media for efficient and traceless purification of compounds with maximal biological integrity [
9]. In the research laboratory, aptamers have been implemented to investigate binding phenomena in, for example, proteomics similar to what was envisioned in proteomics-based biomarkers [
10,
11,
12,
13,
14]. Thus, aptamers as artificial ligands with a variety of unique properties are attractive for a spectrum of functions, from basic research to translational medicine.
Neurodegenerative disorders have been the second leading cause of death and the first leading cause of dementia in the past decade, which affect millions worldwide but lack effective treatment and prevention [
15]. These diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Transmissible Spongiform Encephalopathies (TSE), Huntington’s Disease (HD), and prion protein diseases (PrD), are characterized by the accumulation of misfolded proteins in the CNS resulting from either genetic or environmental materials, as well as both factors.
Current research aims to manage neurodegenerative diseases by genetic intervention [
16,
17], as with siRNA drugs or gene therapy with, for example, adeno-associated virus (AAV) vectors. However, the safety and efficacy of gene therapies continue to involve challenging issues. Monoclonal antibody-based therapies are now available, such as treatment in multiple sclerosis (MS) [
18] and the targeting of amyloid-beta (aducanumab [
19]) for the treatment of AD. These applications have gained FDA approval, despite controversy, indicating that biological molecules can be developed to treat neurological and neurodegenerative disorders, and also reaffirming that it is feasible to treat neurodegenerative diseases by inhibiting protein post-translational modification (PTM), misfolding, and aggregation processes [
20]. These approaches target a common pathological feature of these diseases: the accumulation of misfolded or PTM proteins and the resulting deposition of protein aggregates that appear to be toxic to nerve cells.
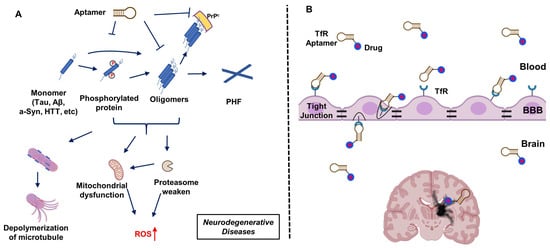
In concept, aptamers, have a number of advantages over antibodies, including lower immunogenicity, smaller physical size, and better permeability [
21]. Aptamers have evolved to bind neurologic disease-relevant proteins, inhibit neurodegenerative events resulting from PTM (e.g., phosphorylation), and/or prevent aggregation (
Figure 1A). On the other hand, the transfer of biological molecules across the blood–brain barrier (BBB) remains the most challenging obstacle to treat brain diseases. On this front, exploiting the transferrin receptor (TfR) binding aptamer moiety provides a potentially viable path to the delivery of neuroactive aptamers or other drug molecules across the BBB (
Figure 1B).
Figure 1. Aptamers applied in the treatment of neurodegenerative diseases. (A) Schematic illustration of aptamer mitigation of damage caused by the aggregation of neuro-related protein monomers (Tau, amyloid-β (Aβ), alpha-synuclein (a-Syn), Huntingtin (HTT)) that are often post-translationally modified or subject to proteolysis. (B) Schematic of the strategy of aptamer transfer across the blood–brain barrier (BBB) via transferrin receptor (TfR) mediated transcytosis. PHF: Poly(hexylene 2,5-furandicarboxylate); ROS: reactive oxygen species; PrPc: cellular prion protein.
2. Aptamers Targeting Neuro-Medically Relevant Targets
Neuroprotein-targeting aptamers may also demonstrate outstanding potential for inhibiting protein aggregation associated with brain neuropathology, paralleling the success of aptamers in protein-binding applications. As discussed above, multiple aptamers have been selected against different CNS-relevant neuroproteins. This section will discuss some recent accomplishments of applying CNS-specific aptamers to the development of targeted neurotherapeutics. These crucial challenges have delayed the clinical translation of aptamers and the adoption of improvements in aptamer-based approaches in brain therapy.
Table 2 shows several DNA or RNA aptamers with good binding to CNS-specific or enriched protein targets, with affinities ranging from µM to nM. These include DNA and RNA aptamers that target β-Amyloid (Aβ) protein, Aβ42 dimer, Aβ40 oligomers, Tau protein for AD, and potentially AD-related disorders such as 4R tauopathies [
72]. Further, α-synuclein protein and Prion protein (PrP) aptamers have been developed for PD and prion protein diseases [
73]. In other studies, Toll-like receptor 4 (TLR4) and the regulator of calcineurin 1 (RCAN1) have been targeted for aptamer development as a potential treatment for Stroke disease [
74] such as Acute ischemic stroke (AIS) [
75,
76]. For Amyotrophic Lateral Sclerosis (ALS), an aptamer has been identified for targeting TAR-DNA-Binding Protein 43 (TDP-43) [
77].
Table 2. Known aptamers targeting neuroproteins or neuro-medicine-relevant proteins or cells.
Our laboratory has used a different strategy that targets other peptides that mimic critical pathological tau phosphorylation sites (Thr181, Ser 202, Thr231, Ser396/Ser404). This work identified an optimized DNA aptamer IT2a [
81]. This epitope-specific DNA aptamer selectively recognizes Tau proteins with promising in vitro ability to inhibit Tau oligomerization and hyperphosphorylation.
In addition to Tau, Takahashi, and colleagues [
85] and Song group [
86] successfully selected RNA aptamers against the Aβ1-40 peptide, showing that the aptamers efficiently inhibit Aβ aggregation in vitro with therapeutic potential for treating AD. More recently, Lida et al. focused on preventing AD by destroying the formation of prion protein-Aβ oligomers [
102]. They identified an anti-prion RNA aptamer, but more in vivo studies are needed to demonstrate its therapeutic ability. Interestingly, the Irie group from Kyoto University reported the first RNA aptamer targeting the toxic dimer of Aβ42 with significant prevention against the formation of Aβ42 aggregates and the related neurotoxicity [
88] (
Table 2).
Observation of oligomers and aggregates formed by disordered alpha-synuclein (α-syn) in neurons has been used to characterize Parkinson’s disease (PD). Similar to PD, the accumulation of aggregated α-synuclein protein in Lewy bodies and Lewy neurites results in dementia with Lewy bodies (DLB). Thus, reducing the aberrant aggregation of intracellular α-syn may prevent the progression of synucleinopathies such as PD and DLB [
111]. The Zhang group has reported the identification of DNA aptamers targeting α-syn with high affinity [
90]. Their aptamers present effective inhibitory ability on α-syn aggregation and promote its degradation in cell-based studies, rescuing cellular defects and showing potential for PD therapy. Other studies have shown different DNA aptamers that bind to α-syn fibrils and inhibit α-syn aggregation in in vitro models of PD and DLB [
92,
93,
94]. Interestingly, Lobanova et al. selected DNA aptamer that binds to fibrillar aggregates of α-syn and Aβ detected in both serum and CSF in PD [
95] (
Table 2)
Exosomes have been employed to carry aptamers targeting α-syn [
112] and deliver them into the PD animal model brain for improved investigation of in vivo effects. The aptamer-loaded exosomes were efficiently delivered into neurons in the animal model, strongly reducing in vivo neuropathological α-syn aggregates and alleviating behavioral deficits. Therefore, the anti-α-syn aptamers are promising therapeutic agents for the clinical treatment of synucleinopathies, such as PD. Further, exosomes have also been modified with aptamers targeting myelin, giving outstanding performance in promoting the remyelination process in mice models. This indicates the potential of preparing aptamer-based nano-medicines for managing sclerosis disease [
113] (
Table 2)
Both myelin basic protein (MBP) and autoantibodies against MBP have been targeted for aptamer development as potential multiple sclerosis (MS) therapy [
114]. For glioma, aptamers have also been identified for CD20+B cells and the U87 glioma cell line/EGFRvIII. Neuro-aptamers target glutamate receptor GLuR1 [
115], as well as the neuronal cell body and neurite-surface-located cell adhesion molecule (L1CAM) [
101,
116]. These neuro-aptamers may also be useful neuroscience research tools (
Table 2)
Besides directly using aptamers as therapeutic agents, some researchers have linked the targeting ability of aptamers with neurotherapy in combination with other therapeutic agents, such as chemotherapy drugs. For example, a Gint4.T aptamer targeting platelet-derived growth factor receptor β has been widely used for glioblastoma neurotherapy. Monaco et al. anchored the aptamers to polymeric nanoparticles to cross the BBB, where they entered glioblastoma cells [
98]. By loading with PI3K-mTOR inhibitor, a promising chemotherapeutic drug for treating glioblastoma, this aptamer-based nano-system demonstrated specific tumor accumulation and mTOR activity inhibition in a mouse model.
Similar studies were carried out by Shi et al. and Wang et al., who built tetrahedral DNA structures loaded with Gint4.T aptamers to facilitate BBB penetration and glioma engagement. These DNA structures were captured with either paclitaxel or doxorubicin to enable anti-glioma therapy [
117,
118] (
Table 2).
3. Applying Experience from Non-CNS Therapeutic Aptamers towards Neuro-Aptamer Development
The potential for using aptamers in therapy was recognized in 1990 when Tuerk and Gold selected an RNA aptamer to target bacteriophage T4 DNA polymerase in the first SELEX experiment [
2]. In the same year, Sullenger et al. reported the inhibitory effect of transactivation response (TAR)-containing sequences (TAR decoys) on HIV-1 viral infection in host cells. These TAR decoys (which perform as if they were aptamers) prevent the Tar protein from binding to the endogenous TAR RNA, inhibiting HIV gene expression and replication. TAR decoy RNA-mediated HIV inhibition was also suggested to be effective against natural HIV isolates despite their hypervariable nature because the replication of SIVmac was also inhibited in cells expressing HIV-1 TAR decoys [
133]. These two independent discoveries suggested that specific nucleic acid sequences have potential for therapies in general.
Since then, aptamers have been studied in preclinical and clinical tests with different strategies employed for therapeutic applications, including aptamers as antagonists or agonists, and targeting ligands conjugated onto the drug carriers. In 2004, the first aptamer therapeutic, Macugen (Pegaptanib) was approved by the FDA, which is presently the only aptamer approved by the US FDA. It is effective as an anti-angiogenic medicine to treat neovascular (wet) age-related macular degeneration (AMD) [
134].
Therapeutic targets for aptamers to date include thrombin [
135] and nucleolin [
136]. In addition, aptamers have been used to treat aging-related disorders [
137], obesity and diabetes mellitus [
138,
139], cardiovascular diseases [
140], infectious diseases [
141,
142], blood coagulation [
143], bone diseases [
144], immunological diseases [
145] and cancers [
146]. Experience from developing therapeutic aptamers for this range of targets, and knowledge regarding aptamer technology derived from this experience [
147], will likely accelerate the development of aptamers toward managing neurological or neurodegenerative disorders.
4. Aptamers Targeting Transferrin Receptor 1 to Facilitate Drug Transport across the BBB
The BBB impedes the entry of blood-borne molecules and is necessary to maintain brain homeostasis. The BBB comprises endothelial cells joined by highly polarized tight junctions and supported by astrocytes and pericytes responsible for the isolation of the brain from peripheral circulation [
148,
149].
The highly restrictive nature of the BBB limits access to most bio-therapeutics, including antibodies, aptamers, and most (~98%) small-molecule drugs to the brain microenvironment [
150]. Aptamers targeting CNS proteins may not cross the BBB unless they are combined with BBB-penetrating agents or unique loading structures, such as neuronal cell-derived exosomes, exploiting the TfR-based transcytosis, or some efficient nano-systems (such as tetrahedra or circular DNA structures and nanoparticles).
Structures implementing soft or solid nanomaterials improve the serum stability brain retention times of aptamers compared with the aptamers alone. This may mitigate the inherent drawbacks of rapid nuclease degradation and rapid renal clearance. This strategy may improve therapeutic efficacies [
151].
Transferrin receptor 1, which is abundant in endothelial cells on the neurovascular material that forms the BBB, is considered a promising target for CNS delivery across the BBB. This has generated interest in developing antibodies [
152] that bind TfR for CNS delivery [
153,
154]. Yu et al. reported the development of a low-affinity monoclonal antibody that binds TfR and therefore can cross the BBB to enhance the delivery of conjugated therapeutic antibodies that bind enzyme β-secretase (BACE1), a target for Alzheimer’s drugs.
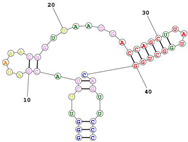
Several TfR DNA and RNA aptamers are now known. Thus, Neufeld et al. first developed the GS24 DNA aptamer (50 nucleotides long [
155]) for its ability to bind mouse transferrin receptor 1 (TfR1) as shown in
Table 3. GS24 was subsequentially truncated and mutated by MacDonald et al. to give a short aptamer, TfRA1, only 14 nucleotides in length [
156]. This team then further exploited a bispecific strategy that conjugated TfRA1 with the epithelial cell adhesion molecule (EpCAM) to treat brain cancer metastases [
157].
Table 3. Currently published transferrin receptor aptamers.
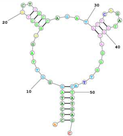
It is important to note that GS24 and TfRA1 are specific to mouse TfR and do not cross-react with their human counterparts. Maier et al. developed a TfR-specific RNA aptamer (WAZ), which targets the apical domain of the human transferrin receptor (hTfR) and does not interfere with transferrin binding. They propose that this is a critical feature for medical applications, as this aptamer will not interfere with the physiological functions of transferrin binding and uptake into the target cells (or through the BBB).
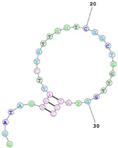
Last, Wu et al. reported a DNA aptamer (XQ-2d) selected against pancreatic ductal adenocarcinoma using the cell SELEX strategy [
159]. The team subsequently found that its cellular target for XQ-2d was cell surface-bound CD 71, also known as TrfR1 [
160].
This entry is adapted from the peer-reviewed paper 10.3390/molecules29051124