Similarly to AAAs, TAAs are characterized by an extensive remodeling of the ECM [
]. Endothelial dysfunction is also present in TAAs, but the formation of fibro-atherosclerotic plaque is rarely evidenced [
]. In addition, endothelial cells can undergo endothelial-to-mesenchymal transition (End-MT), a process in which cells switch from an endothelial to a mesenchymal phenotype, losing cell-to-cell contact and cell polarity [
]. The main pathway responsible for the End-MT is the canonical Transforming Growth Factor β (TGF-β) signaling in association with NOTCH and Wnt/β catenin signaling [
]. End-Mt has been observed in sporadic TAAs but appears to be more accentuated in MFS TAAs [
]. At the same time, SMCs in the tunica media can dedifferentiate in myofibroblasts, switching from a contractile phenotype to a proliferative/synthetic one. Myofibroblasts deposit alcianophilic material like Glycosaminoglycans (GAGs) and collagen, determining fibrosis [
]. This process is well evidenced in sporadic TAAs and strongly exacerbated in MFS TAAs [
]. SMCs can also acquire an osteoblast-like phenotype, promoting calcification, especially in BAV TAAs [
]. Those alterations are associated with the deregulation of NOTCH and BMP signaling [
].
In the last few decades, many studies have demonstrated that about 97% of the human genome consists of non-coding sequences that are not transcribed in mRNAs but in non-coding RNAs [
31]. Non-coding RNAs regulate gene expression at the post-transcriptional level [
32]. miRNAs are a class of non-coding RNAs, which act as epigenetic regulators without affecting the chromatin architecture [
33]. In the human genome, miRNAs are encoded by introns of "host genes" and also enriched within unique miRNA clusters [
33]. miRNA biogenesis starts with RNA polymerases II/III, which transcribe a primary miRNA (pri-miRNA) molecule from the intergenic or intragenic region [
32]. Successively, pri-miRNAs are processed by a multi-component microprocessor complex (Drosha/DGCR8) to precursor miRNAs (pre-miRNAs) with specific hairpin structures [
32]. Pre-miRNAs are exported from the nucleus to the cytoplasm via the transport factor exportin-5 using GTP as a cofactor [
32]. Within the cytoplasm, pre-miRNAs are released from exportin-5 following GTP hydrolysis and are further processed by an RNase III, called “Dicer”, generating double-stranded miRNAs of approximately 22 nucleotides [
32]. This product is incorporated into the RNA-induced silencing complex multiprotein complex (RISC) in which a helicase ensures that only one of the miRNA duplex strands remains in the complex to control the post-transcriptional expression of target genes [
34]. miRNAs are known as negative regulators of gene expression [
34]. Precisely, miRNAs induce the cleavage and degradation of target mRNAs or the inhibition of the translation process [
34]. miRNAs have been demonstrated to play a critical role in several physiological processes, such as cell proliferation, apoptosis, fat metabolism, neuronal development, cell differentiation, hormone secretion and the development of multiple diseases [
35]. In the last few years, many studies have reported an aberrant miRNA expression in different cardiovascular diseases such as atherosclerosis, cardiac remodeling, myocardial infarction and aneurysms [
36].
5. The Regulatory Role of miRNAs in AAAs
Different studies have reported that inflammation, endothelial dysfunction, ECM remodeling and SMC proliferation/apoptosis, which characterize the AAA aortic wall, are associated with specific miRNA deregulations [
39]. ECM proteins such as collagen and elastin are mainly produced by SMCs, and the apoptosis of those cells has been demonstrated to be implicated in the development of AAA [
40]. It has been reported that SMAD3, a key intracellular mediator of the fibrotic process [
41], is down-regulated in AAA [
42,
43]. It was also found that miR-195, an important regulator of ECM proteins, was up-regulated in AAA patients [
44]. Bioinformatics analysis revealed that miR-195 has a binding site in the 3′-UTR for SMAD3, and their interaction was confirmed by in vitro studies [
45]. miR-195 overexpression in vitro inhibited SMC proliferation, inducing apoptosis, whereas SMAD3 overexpression blocked those effects [
45]. Therefore, miR-195 displays a crucial role in AAA pathogenesis and represents a potential therapeutic target [
45].
As mentioned above, AAA is characterized by an increased degree of calcification, which can lead to rupture [
46]. During AAA progression, SMCs switch from the contractile to the synthetic phenotype and synthetize osteogenic factors, such as the osteogenic transcription factor Runt-related gene (RUNX). The latter is functionally associated with SMAD2/3, which, in turn, are activated by angiotensin II (AngII) signaling [
47]. The activation of the SMAD-RUNX2 signaling pathway induces osteogenic differentiation of SMCs [
47]. It has been demonstrated that miR-424 has a key role in cardiovascular pathologies. miR-424 is also called “osteomir” as it regulates vascular calcification [
48]. Bioinformatics analysis revealed that AAA patients express high levels of
RUNX2 and exhibit down-regulation of miR-424 compared with control subjects [
47].
Another miRNA, reported to be involved in the chronic inflammation that characterizes the AAA aortic wall, is miR-33-5p [
54]. Some studies reported that this miRNA regulates the innate immune response by the adenosine triphosphate-binding cassette transporter A1 (ABCA1) [
55,
56]. ABCA1 is a protein that transports free cholesterol and phospholipids from intracellular compartments to the cell membrane by using ATP as a source of energy, so this protein is fundamental in macrophage cholesterol efflux and reverse cholesterol transport [
57,
58]. It has been reported that ABCA1 acts as an anti-inflammatory receptor, and the enhancement of its function could be a beneficial therapeutic approach [
59].
Another study correlated miR-21 expression to inflammatory response and aortic remodeling in AAA. Precisely, Yu et al. investigated the effects of dexmedetomidine (Dex) on miR-21 expression [
60]. It has been reported that Dex suppresses the activities of inflammatory mediators and maintains a balanced myocardial function and coronary blood flow [
61]. miR-21 is reported to control the inflammatory responses, ECM remodeling and lipid accumulation in cerebral aneurysms [
62,
63]. Moreover, it has been demonstrated that miR-21 inhibition blocks AAA development. Programmed cell death 4 (
PDCD4), an inflammation- and apoptosis-related gene, has been proven to be a gene target of miR-21 [
64,
65]. In order to evaluate the effects of Dex on miR-21 expression and consequently on AAA progression, rat models of AAA were injected with Dex. The authors discovered that Dex administration in AAA rat models down-regulated the expression of inflammatory factors and MMPs as well as up-regulating miR-21 expression [
60].
6. The Regulatory Role of miRNAs in Sporadic TAAs
As previously reported, TAA formation is associated with progressive pathological remodeling of the aortic wall that leads to structural parietal degeneration, with rearrangement of hemodynamic loads, and finally rupture [
66,
67]. During this remodeling, both endothelial cells and SMCs undergo phenotypic changes in response to pathogenetic stimuli [
22,
68,
69]. Recently, miRNA deregulation has been reported to be associated with vascular cell phenotypical changes. The phenotypic switching of SMCs from a contractile to a synthetic phenotype has been suggested to be involved in the development of aortic aneurysm and its dissection [
68,
69]. As already mentioned, aortic SMCs of sporadic TAA generally switch into a myofibroblast phenotype with the increased collagen synthesis and consequent aortic fibrosis [
70,
71]. During this process, SMCs reduced the expression of functional markers such as smooth muscle 22 α (SM22α), smooth muscle cell-specific myosin heavy chain (MYH11) and α-smooth muscle actin (α-SMA) [
68]. However, the molecular mechanisms underlying the SMC phenotypic switch is not completely understood. The analysis of aortic tissues derived from patients with severe TAA evidenced a strong miR-335-5p down-regulation and Specificity Protein 1 (
SP1) up-regulation; the latter is involved in SMC proliferation and phenotype switching [
72]. However, cultured human SMCs overexpressing miR-335-5p showed
SP1 down-regulation with reduced proliferation and migration as well as increased expression of contractile markers, such as SM22α, α-SMA and CNN1 [
72]. In addition, the authors documented
SP1 as a gene target of miR-335-5p. In a mouse model of aortic dissection, the administration of miR-335-5p clearly suppressed aorta dilatation and vascular media degeneration [
72]. Aberrant down-regulation of miR-134-5p has been evidenced in aortic tissues derived from sporadic TAA patients [
73]. In vitro studies, on aortic SMCs, revealed that miR-134-5p overexpression promoted differentiation and expression of contractile markers, suggesting a crucial role of miRNA in aortic SMC homeostasis [
73]. PDGF and TGFβ are involved in SMC differentiation, vascular remodeling and aortic aneurysms [
74,
75].
Endothelial cells play a fundamental role during pathological aortic aneurysmatic remodeling [
22]. Several studies identified the aberrant expression of different miRNAs in TAA and their role in the regulation of endothelial cell phenotyping and functions [
77]. The activation of endoplasmic reticulum stress (ERS) contributes to the pathogenesis of cardiovascular diseases, in particular endothelial dysfunction [
78,
79,
80]. The mechanism through which ERS mediates vascular cell dysfunction is not completely understood. miRNAs regulate the ERS response by targeting specific genes [
81]. In particular, miR-204 seems to be associated with ERS by targeting sirtuin1 lysine deacetylase (
SIRT1) [
82,
83]. Kassan et al. investigated the role of miR-204 in ERS and endothelial dysfunction by targeting
SIRT1 [
84]. Overexpression of miR-204 in HUVECs induced ERS by up-regulation of specific stress markers, such as glucose-regulated protein, C/-EBP homologous protein and Activating Transcription Factor 6, as well as phosphorylation of PKR-like ER kinase and eukaryotic initiation factor 2.
7. miRNA Regulation of Vascular Cell Phenotype in Genetic TAAs
As reported above, TAAs are, in some cases, associated with genetic mutations [
87]. BAV (bicuspid aortic valve) is a congenital cardiovascular malformation leading to an increased risk for severe cardiovascular events, such as TAA [
88,
89,
90]. The BAV condition is associated with different genetic mutations including the
NOTCH1,
TGFBR2, FBN1,
SMAD6, GATA5 and
GATA6 genes [
88,
89,
90]. Several studies described the differential expression of miRNAs between BAV and TAV (tricuspid aortic valve) patients [
91]. Some studies reported that BAV aortopathy is also characterized by a lower expression of End-Mt markers compared with sporadic TAAs [
8,
92]. However, some studies demonstrated that, before dilation, BAV aortas showed an activation of the End-Mt process [
93]. Using a systemic biological approach, a strong association between the miR-200 family, which targets the End-Mt transcription factors zinc-finger E homeobox-binding transcription factors 1 and 2 (
ZEB1 and
ZEB2) [
94,
95], with the BAV signaling network was highlighted [
93].
The differential expression of ERG and its transcription factor miR-126-5p has also been demonstrated, in the vascular phenotypic changes occurring in BAV and TAV aortas [
8]. The expression levels of the protein ERG and miR-126-5p were up-regulated in TAA samples derived from BAV compared to TAV patients. Moreover, this up-regulation in BAV TAA was shown to be associated with a down-regulation of SMAD2/3 proteins [
8], whose activation induces End-MT [
96]. Therefore, the down-regulation of SMAD2/3 could explain the different phenotypic changes observed in the endothelium of BAV TAA. Similarly, the tunica media of BAV TAA showed an up-regulation of ERG and miR-126-5p in association with an evident aortic calcification compared with TAV tunica media; the latter were mainly characterized by a marked fibrosis [
8].
8. Conclusions
Aortic aneurysms remain a serious health concern with many clinical complications as the associated ruptures can cause significant morbidity and mortality. The onset and development of AAA and TAA are associated with different risk factors. AAA is generally associated with atherosclerosis, hypertension and aging, whereas TAA frequently occurs in patients with genetic diseases. Both types of aortic aneurysms are characterized by pathological aortic remodeling. In that light, AAA shows a more marked inflammatory parietal response compared with TAA. miRNAs have been identified to be involved in the regulation of vascular cell phenotypic transformation, inflammation and SMC apoptosis. In AAA, miR-195 and miR-21 deregulation are associated with SMC apoptosis; miR-424 down-regulation is associated with calcification of the aortic wall; miR-33b and miR-33-5p deregulation is involved in the parietal inflammatory response. In TAA, miRNA deregulation induces a vascular cell phenotype switch. In sporadic TAA, miR-335-5p, miR-134, miR-155b-5p, miR-122-3p and miR-23b-5p down-regulation lead to a reduction in SMC cytoplasmic contractile cytoskeletal filaments with a switch into a synthetic phenotype promoting medial fibrosis. In TAA, endothelial cells characteristically lose physiological endothelial markers, with an End-Mt phenotypic switch and consequent endothelial dysfunction. For genetic TAA, MFS TAA shares histopathological features with sporadic TAA such as End-Mt and fibrosis, but in a more accentuated manner. Deregulation of specific miRNAs such as miR-29, miR-122 and miR-632 plays a key role in the regulation of the phenotypical cell changes and parietal remodeling observed in MFS TAA. Aortic remodeling in BAV TAA differs from sporadic and MFS TAAs, and miRNAs appear crucial in the regulation of aortic remodeling. Deregulation of miR-126, miR-200 and miR-423-5p seems to inhibit the End-Mt process and favor the calcification of the tunica media observed in BAV TAA. Schematic representations of the phenotypic alterations and aortic remodeling of AAA and TAA regulated by miRNAs are shown in Figure 1 and Figure 2.
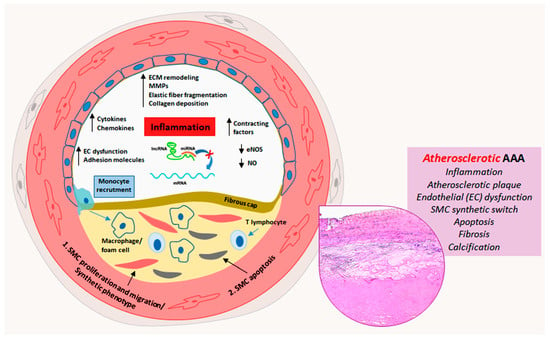
Figure 1. Schematic representation of miRNA deregulation-related pathogenic mechanisms in atherosclerotic abdominal aortic aneurysms. Atherosclerotic process prevails in the progression of abdominal aortic aneurysm (AAA) and is characterized at least in part by miRNA deregulation-driven endothelial dysfunction, inflammation, smooth muscle cell (SMC) proliferation, migration and apoptosis, fibroatheromatous plaque formation and thrombosis, with medial fibrosis and calcification (insert, Hematoxylin and Eosin staining).
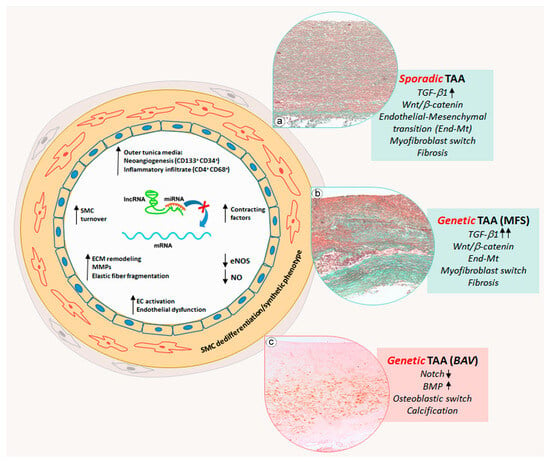
Figure 2. Schematic representation of miRNA deregulation-related pathogenic mechanisms in thoracic aortic aneurysms. Thoracic aortic aneurysms (TAAs) are sporadic or frequently related to genetic diseases. Sporadic and Marfan syndrome disease (MFS) TAAs show an aortic cell differentiative process, in which endothelial cells switch into a mesenchymal phenotype, whereas medial SMCs switch into a myofibroblastic phenotype and synthetize collagen and glycosaminoglycan with consequent aortic degeneration and fibrosis (inserts (a,b), Masson’s Trichrome Goldner staining). Those processes are precocious, much more marked and strongly accentuated in MFS TAAs, in which TGF-β signaling is hyperactivated. Genetic bicuspid aortic valve (BAV) TAA displays a specific degenerative process, in which SMCs dedifferentiate into osteoblast-like cells promoting medial calcification (insert (c), Alizarin Red staining) by miRNA deregulation-mediated activation of NOTCH and BMP signaling.