Low-density lipoprotein (LDL) cholesterol and serum total cholesterol (TC) levels are both linked to a high risk of ischemic heart disease found in people at a young age and people at low risk of coronary heart disease [
1,
2,
3,
4]. With the increasing incidence of hypercholesterolemia and associated cardiovascular risk, safe, effective, and inexpensive therapeutic approaches were developed to manage such affections [
5]. However, over time, it is necessary to understand the extent of blood lipid-induced complications and how they can be managed, which will allow a clear understanding of coronary risk factors among populations [
4,
5,
6,
7]. Such studies will also help to identify opportunities to reduce coronary heart risk [
8]. According to the World Health Organization (WHO) guidelines, in Kazakhstan, the age-standardized prevalence of total cholesterol increase (≥6.2 mmol/L) was estimated at 12%, which is close to the Russian Federation (15%), but much higher than other Central Asian countries. For instance, Turkmenistan estimated the age-standardized prevalence of total cholesterol increase as the highest, i.e., 8%, and the minimum was 5% in Tajikistan. However, in Central Asian countries, it was estimated to be much lower than in other developed countries, such as Germany (25%) and England (22%) [
9].
Milk and its products represent an essential part of a healthy balanced diet, rich in proteins and micronutrients [
21,
22]. Nonetheless, there was a misconception regarding dairy products consumption in the past, linked to the premise that they are high-risk factors for cardiovascular diseases and create adverse effects on human health due to the presence of a marked content of saturated fatty acids [
23]. Though, this claim was proved wrong by the fact that dairy products have functional components (calcium, milk proteins, and phospholipids) of high nutritional value, which are efficient in lowering the risk of cardiovascular disease. Their health benefit is attributed to their probiotic effects and the ability to modulate the lipoproteins metabolism [
24,
25].
2. Hypercholesterolemia
2.1. Low-Density Lipoprotein-Cholesterol and the Concept of Hypercholesterolemia
Hypercholesterolemia (HC) is related to an elevated level of plasma low-density lipoprotein-cholesterol (LDL-C), as plasma LDL is the chief carrier of cholesterol [
33]. The gut absorbs dietary cholesterol primarily packed in chylomicrons(triglyceride-rich); these chylomicrons first break down with the help of lipoprotein lipase (LPL), then the fatty acids and monoglycerides released are further supplied to the adipose tissue and muscles. In contrast, the remnant of chylomicron passes through the liver. Then liver produces very-low-density lipoproteins (VLDL), from which they are exuded into the bloodstream.VLDL becomes enlarged due to the presence of triglycerides in it. As these triglycerides are unable to accommodate themselves in VLDL, instead they start to aggregate themselves in the liver resulting in the development of fatty liver disease.LPL metabolizes VLDL and produces intermediate-density lipoproteins (IDL). Later, it becomesconverted to LDL. The liver also produces high-density lipoprotein (HDL) particles from cholesterol, incorporating cholesteryl esters released from peripheral tissues. In this way, HDL becomesinvolved in the reverse transport of cholesterol from peripheral tissues to the liver. Cholesteryl ester-laden HDL particles are moved to LDLs via cholesteryl ester transfer protein (CETP). Then, these cholesteryl esters are carted via LDL particles, which are absorbed by the liver and peripheral tissues (to a lesser extent). The LDL uptakes, as well as degradation in the liver, rely on LDL particles’ binding onto cell surface receptors (LDL-receptors) of hepatocytes. These receptors are particularly connected to apolipoprotein B (apoB) on the LDL particle. Then, the LDL: LDL-receptor complex is internalized via endocytosis. The whole internalization process is reconciled through the LDL receptor adaptor protein. These receptor molecules becomerecycled, and the LDL particles undergo lysosomal degradation. A protein expressed in hepatocytes, the proprotein convertase subtilisin/kexin type 9 (PCSK9), is believed to play a vital role in the catabolism of LDL-receptors [
34].
2.2. Epidemiology
Among the Indian population, the prevalence of HC differs between rural (10–15%) and urban (25–30%) populations [
36]. To date, different data-based studies were performed on the Indian population, including the India Migration Study (
n = 1983), India Heart Watch (
n = 6123), Indian Industrial Population Surveillance Study (
n = 10,442), Indian Women Health Study (
n = 4624), Indian Council of Medical Research (ICMR) Integrated Disease Surveillance Project (urban
n = 15,223, slum/periurbann = 15,751, rural
n = 13,517), a nationwide industry-sponsored FitHeart Study (
n = 46,919) and the INDIAB study (
n = 2042) [
37,
38,
39,
40,
41,
42]. Among those, Al-Kharj of Saudi Arabia showed a positive association between the prevalence of HC and increasing age in the general population [
43].
2.3. Investigation and Laboratory Assessment
Currently, there are various therapeutic options to manage HC; however, before starting the treatment for HC, clinical examination, detailed history, and basic laboratory tests are needed to properly diagnose the causes of secondary HC, other complications related to atherosclerosis, and primary manifestations of lipoprotein changes [
35]. In this sense, a family as well as occupational history, including the history of alcohol consumption, smoking, and dietary preferences, will help to determine the level to which these factors contribute to the risk of cardiovascular disease and/or HC and determine the patient’s ability to change lifestyle [
35]. Regarding physical examination, it should include waist circumference, body weight, blood pressure, height, and a search for the presence of xanthoma [
35]. Finally, family history is also an important factor in helping cascade testing at the primary stage of HC [
55].
Moreover, during HC screening, it is advisable to obtain blood samples post-fasting for a minimum period of 10 h to avoid the postprandial contribution of plasma triglycerides [
35]. If the plasma triglycerides range is in the limit of up to 4.5 mmol/L, the LDL cholesterol concentration can be calculated using the Friedewald formula:
Low density lipoprotein cholesterol = total serum cholesterol—(HDL cholesterol + (serum triglycerides/2.2)).
Non-HDL cholesterol = total cholesterol − HDL cholesterol.
Non-HDL cholesterol may act as a target for statin treatment if LDL-C cannot be measured owing to hypertriglyceridemia. The HDL cholesterol and total cholesterol: HDL ratio values help evaluate the risk of cardiovascular disease, but LDL cholesterol can be used as a therapeutic target whenever possible. Non-HDL cholesterol targets are 0.75 mmol/L more than the LDL cholesterol target. In addition, to assess the risk of cardiovascular disease, it is also important to determine fasting glucose concentration, and before starting lipid-lowering treatment, some parameters must be measured, such as creatine kinase activity, liver function tests, dipstick urine protein, and serum creatinine [
35].
For routine assessment, many testing laboratories use handheld point-of-care testing (POCT) devices and automated analyzers depends on enzymatic assays a commercialised cholesterol quantitation assay kits [
56,
57]. Point-of-care testing (POCT) gives quick results, with simplefunction, making it more suitable for people screening tests. POCT clinical application has been found efficient in raising awareness regarding significance of lipid levels to prevent future stroke events and CVD [
58]. The CardioChek PA analyzer (PTS Diagnostics) is a handy whole blood test system including a single test strip to determinetriglycerides (TG)HDL cholesterol (HDL-C) and total cholesterol (TC) [
59]. In Brazil, the CardioChek PA analyzer used by health professional workers is well recommended for the proposed screening programs; the diagnostic performance is appropriate for use as part of national health services, providing quick and accurate results.
2.4. Treatment
2.4.1. Non-Pharmacological Therapy
There are various non-pharmacological options to manage HC, and dietary therapy is one of the most important ones. Among others, this approach’s primary goals are to progressively lessen the intake of total fat, saturated fats (saturated fatty acids), cholesterol and attain desirable body weight. For example, it is advisable to reduce the intake of saturated fat daily up to 7% of calories, the daily intake of total fat to 25 to 35% of calories, to limit the dietary cholesterol quantity to no more than 200 mg/day; incite the intake of soluble fiber to 20 to 30 g/day, widely abundant in oats, beans, peas, and in some specific fruits [
60]. Dietary fibers containing insoluble and soluble fibers attained from plants involvinglignin and non-digestible carbohydrates. Soluble fibers consist of viscous fibers such asmucilage, gum, β-glucans, pectin, fructans (inulin, fructooligosaccharides), and hemicellulose (non-viscous fibers). The insoluble fibers consist of some lignin, cellulose, and hemicellulose [
61]. While both insoluble and soluble fibers are not digestible and can be fermented by bacteria with the help of enzymes to hydrolyze the fiber, soluble fibers are more easily fermented by the gut bacteria and have prebiotic functions providing a short-chain fatty acids source. As such, short-chain fatty acids are quickly absorbed by the large intestine, can be oxidized, and used for the production of energy. Short-chain fatty acids suchas propionic acid absorption have been found to reducethe synthesis of cholesterol in the liver, leading to lessening the blood cholesterol and enhanced water and sodium absorption into the colonic mucosal cells [
62,
63]. Alternatively, functional fibers are nondigestible carbohydrates that are either synthesized or extracted (isolated) and manufactured, to confer valuable health effects. Functional fibers are chitosan, β-glucans, chitins, cellulose, gums, fructans, resistant dextrins, lignin, polydextrose, pectin, and resistant starches, polyols, and psylliums [
64].
There are numerous studiescarried outonmetabolism diseases to check the effect of oat β-glucan [
65]. A meta-analysis that included 17 randomized controlled trials (RCTs) (916 hypercholesterolemic patients) displayed that consumption of β-glucan significantly reduced LDL-C (−0.21 mmol/L (8.1 mg/dL)) [
66]. In a randomized, single-blind, wheat bran–controlled study, it revealed that within 8 h if 11 g oat bran β-glucantaken it nearly doubled the plasma secretion of bile acids and reduces serum cholesterol by measuring the metabolite 7-hydroxy-4-cholesten-3-one in the plasma [
67].
It is also expected the inclusion of plant stanols or sterols in the diet (2 to 3 g daily) plus vegetable oils. Other target food items used in reducing total cholesterol include cold-water fishes, such as salmon, sardines, and mackerel, which have rich omega-3 fatty acid contents, widely recognized for lowering triglyceride levels. Similarly, soybeans present in soy, nuts, tofu, and various meat items have a potent antioxidant effect that can help in lowering LDL-C [
60].
2.4.2. Pharmacological Therapy
Among the various drugs available to manage HC, HMG-CoA reductase inhibitors, bile acid resins, fibric acids, niacin, and ezetimibe, are the most often used.
2.4.3. Herbal Treatment
It is widely recognized that Mother Nature has a plethora of potential. With a broad spectrum of activity, a systematic review has suggested that medicinal plants are also rich sources of compounds with anti-HC action rather than saturated fat; plant sterols containing drinks and margarine or stanols can reduce the plasma cholesterol up to 10% [
68].
3. Yogurt
3.1. Concept, History, and Health Effects
Yogurt (also spelled “yoghourt” or “yoghurt”) is a fermented milk-based product believed by some regulatory agencies worldwide as lactose-free and defined explicitly by containing
Streptococcus thermophilus and
Lactobacillus bulgaricus viable bacterial strains [
69]. In ancient times, the use of yogurt for food purposes was recognized by other names over the millennia: cuajada (Spain), mast (Iran), katyk (Armenia), matsoni (Georgia, Russia, and Japan),roba (Sudan), zabadi (Egypt), laban (Iraq and Lebanon), dahi (India), coalhada (Portugal), leben raib (Saudi Arabia), iogurte (Brazil) and dovga (Azerbaijan). Indeed, it has been reported that milk-based products were added to the human diet near around 10,000–5000 BC, with the help of domestic milk-producing animals (such asgoats, cows, yaks, sheep, and camels, as well as buffalo and horses) [
70].
Indian Ayurveda dated back approx. 6000 BC, about the health-related benefits of having fermented milk-based items [
69]. These days, around 700 different cheeses and yogurt items are consumed as part of Indian food. Yogurt was well known in the ROman and Greek empires, and the Greeks were the first to mention it in written references in 100 BC, noting the use of yogurt by barbarous nations [
69]. In the book Bible, Abraham owed his long life and fecundity to consuming yogurt, as is referenced in the description of the “Land of Milk and Honey,” which several historians claim refers to yogurt [
69].
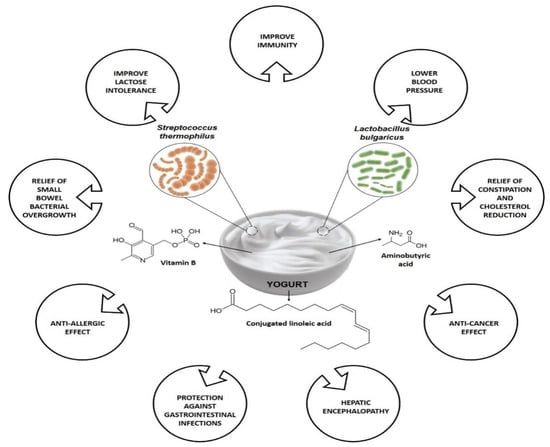
Figure 1 summarizes some of the health benefits associated with yogurt consumption. Both yogurts’ bacterial and non-bacterial components are believed to play health-promoting effects in human health, specifically on the host’s immune system. Among other constituents, bioactive peptides, conjugated linoleic acid (CLA), group B vitamins, c-aminobutyric acid are a few metabolites are found in yogurt, and where the dairy products matrix, bioactive metabolites (i.e., exopolysaccharides and peptides) secreted throughout fermentation along with the active enzymes, fermentation process, are responsible for the health benefits of yogurt [
71,
72]. Based on fat content, the three most important types of yogurts currently available are low-fat, regular, and non-fat yogurts. Low-fat yogurts are made from partially skim or low-fat milk, whereas regular yogurt is formed from full-fat milk. Skim milk is also used for non-fat yogurt [
73].
Figure 1. Variety of health benefits of yogurt consumption.
3.2. Concept of Food Enrichment
An enriched food refers to the food where nutrients have been added to supplement the product with nutrients that are usually present in the original form, but were removed during processing. For example, white bread is a commonly consumed enriched food where some specific vitamins are added because the bleaching process, depletes them [
75].
In a broad sense, despite both enrichment and fortification refer to the addition of nutrients in food, the accurate definition varies. The WHO and the Food and Agricultural Organization of the United Nations (FAO) identifies the term fortification as “
the practicing deliberately increasing the essential micronutrient content, i.e., minerals (including trace elements) and vitamins in food irrespective of whether the nutritional components were primarily present before processing in food or not, so this is to enhance the quality of the nutrients food supply and to avail public health benefit with least health risk”, whereas the term enrichment is defined as
“synonymous with fortification and refers to the adding micronutrients in food which are removed during the time of processing” [
75].
3.3. Phyto-Enrichment of Yogurt
Plants produce a broad pool of secondary metabolites that, among other functions, help them to adapt to various types of harsh environments, and defend them from different pests and microbial attacks, and resist all kinds of abiotic and biotic stresses. In this broad group of secondary metabolites, phenolic compounds have gained significant attention these days because of their anti-mutagenic, antioxidant, anti-clotting, and anti-inflammatory potential, which has been directly linked to their ability to decrease the risk of cardiovascular diseases and cancer development [
76,
77,
78].
Phenolic compounds are the primary dietary source found in fruits [
79]. It has been increasingly underlined that fruit juices, extracts, and powders show different biological activities, owing to which they have been used as functional ingredients in the food industry, such as dairy food industries [
80,
81]. Nonetheless, it is also worthy to note that the seasonal variation in vegetables and fruit production and the high demand for fresh fruits in the market have led researchers to explore alternative sources and strategies for the bioproduction of naturally-occurring bioactive compounds similar to phenolic acids and anthocyanins [
82].
In this way, several foodstuffs have also been used, and specifically, yogurt is a well renowned fermented dairy-based product. Despite its nutritional features and significance in the human diet, it is not being used as a chief source of phenolic compounds [
87]. In dairy food products, the amount of phenolics is minimal due to cattle feed, including a low quantity of phenolics given, contamination of food production equipment with other sanitizing agents, and bacterial decomposition in milk proteins. Hence, some plant-derived additives have been incorporated to raise the phenolic content of yogurt, including the addition of four types of grape callus and grape varieties as functional ingredients [
87,
88].
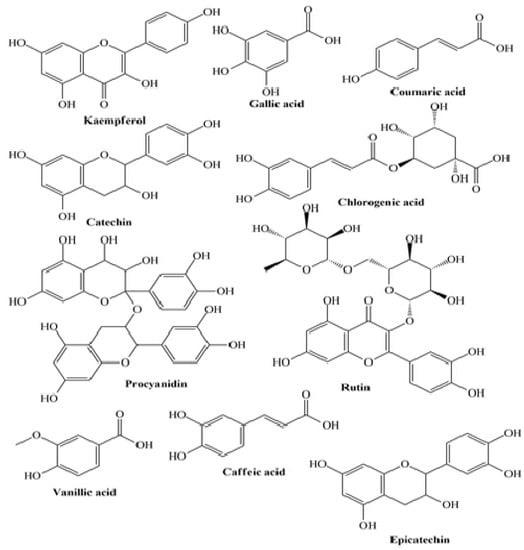
Jaster et al. revealed that anthocyanins present in strawberries-enriched yogurts were directly correlated with a higher antioxidant activity (ABTS) on the 1st and 7th day of storage for control and fortified yogurt, respectively [
90]. A similar trend was stated using the DPPH method, indicating a strong correlation between the antioxidant activity and the anthocyanins content for control and fortified yogurts on the 1st and 7th day, respectively. Marand et al. revealed that after 14 days of storage, the total phenolic content in flaxseed enriched yogurt was higher than that of control yogurt, attributed to the phenolic compounds: ρ-coumaric acid, lignans, ferulic acid, and hydroxycinnamic acid derivatives [
98] (
Figure 2).
Figure 2. Some plant-derived phytocompounds present in enriched yogurt.
4. Phyto-Enriched Yogurt and Hypercholesterolemia Management: Clinical Evidence
There has been an increasing interest in addressing the impact of phyto-enriched yogurt in hypercholesterolemia management in clinical studies. For example, Buyuktuncer et al., in a double-blind, placebo-controlled randomized study for 4 weeks, given to the control group (
n = 35) a placebo yogurt and the intervention (
n = 35) group consumed low-fat yogurt (115 g) with 1.9 g/day plant stanols as esters [
112]. The main findings were a marked decrease in LDL cholesterol (6.3%), serum total cholesterol (4.6%), and non-HDL cholesterol (6.2%) levels from baseline in the intervention group as compared to the control. Insignificant changes were observed at the anthropometric level during the intervention. Similarly, Párraga-Martínez et al. conducted a double-blind, placebo-controlled study involving 182 adults diagnosed with HC [
113]. The authors defined two study groups: the intervention group orally received 2 g of plant stanols containing yogurt drink (
n = 91), and the control group received an un-supplemented yogurt (
n = 91). At 12 months, the lipid profile, defined as the primary endpoint, was changed. In addition, the authors found a >10% reduction in LDL-C in the intervention group (RR 1.7; 95% CI 1.1–2.7). Similarly, Vásquez-Trespalacios et al. revealed in their study that the consumption of a yogurt drink (regular) supplemented with 4 g stanols (plant origin) added as esters compared to yogurt drink (Benecol
®, Colanta) promoted a significant reduction in LDL-C and total cholesterol [
114]. Doornbos et al. revealed that as compared to a placebo, a single-dose yogurt drink (100 g) taken with a meal regardless of its fat content led to a marked decrease in LDL-C [
115].