Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Clinical Neurology
|
Health Care Sciences & Services
Cardiac arrest occurs as a result of a sudden stop of the heartbeat and its mechanical activity, which causes cessation of systemic circulation and blood flow in the brain, which triggers global brain ischemia. Brain neuropathology after cardiac arrest includes primary ischemic injury and secondary reperfusion injury, which occur sequentially, acutely during cardiac arrest and resuscitation, and chronically in the post-resuscitation stag.
- cardiac arrest
- cerebral ischemia
- reperfusion
1. Cardiac Arrest
Cardiac arrest occurs as a result of a sudden stop of the heartbeat and its mechanical activity, which causes cessation of systemic circulation and blood flow in the brain, which triggers global brain ischemia [1,2]. A comprehensive analysis of studies conducted in Europe, China, and the USA showed that the average age of patients who suffered cardiac arrest was 60–66 years, and 58–81% of them were men [3,4,5]. However, rates of mortality, depression, anxiety, and cognitive impairment were higher in women than in men [6,7]. The incidence of cardiac arrest worldwide exceeds 3.7 million annually [8,9]. Among others, in Europe there are 275,000, in the USA 450,000, in Taiwan 10,000, of whom about 10–15% survive until discharge from hospital [10,11,12,13]. The annual incidence of in-hospital cardiac arrest is estimated at 35–55 per 100,000 in Europe and the USA and 28–43 per 100,000 in Asia [9,14,15,16]. In Europe, based on data from the EuReCa ONE and EuReCa TWO studies, the annual incidence of out-of-hospital cardiac arrest was estimated at 84 per 100,000 patients and 89 per 100,000 patients, respectively [17,18], with a moderate average survival rate of 8% [18,19]. In European countries, it is estimated that 56–62 people per 100,000 are treated by emergency services for out-of-hospital cardiac arrest each year [15,18]. In Latin America and Colombia, the epidemiological characteristics and treatment outcomes of patients with out-of-hospital cardiac arrest are similar to those described in the world literature [20]. Similar statistics apply to the United States, Australia, and New Zealand [21,22]. Generally, the return of spontaneous circulation after cardiac arrest ranges from 33.7 to 35.5% [5,23]. Male gender and advanced age have been shown to be independent factors for failure to return spontaneous circulation after cardiac arrest [3,4,5].
Patients who are successfully resuscitated suffer from cerebral hypoxia and ischemia, which is the main cause of side-effects and death after admission to the intensive care unit [24]. Brain damage after cardiac arrest is the leading cause of death in patients resuscitated after cardiac arrest and the leading cause of long-term disability in those who survive the acute phase [25,26]. Therefore, cardiopulmonary resuscitation is the most important therapeutic activity that restores life to patients after cardiac arrest [1,2]. Despite progress in the use of cardiopulmonary resuscitation and targeted brain temperature control by lowering the head temperature to 32–34 °C [12,27,28,29], only 10–15% of patients after cardiac arrest survive until discharge from the hospital [12,13]. Therefore, cardiac arrest and cardiopulmonary resuscitation constitute a serious challenge for emergency physicians around the world and their actions are crucial for patients’ survival. Two key prognostic indicators that are extremely important in post-cardiac arrest are the return of spontaneous systemic circulation and the patient’s neurological status after resuscitation. Unfortunately, cardiac arrest is characterized by a high incidence, limited return of spontaneous systemic circulation, poor neurological outcomes, and poor survival to discharge. Despite significant progress in pre-hospital and in-hospital care, sudden cardiac arrest is still characterized by high morbidity and mortality. Therefore, the impact of cardiac arrest on the quality of life is of great importance worldwide.
2. Brain Neurodegeneration after Cardiac Arrest
Brain neuropathology after cardiac arrest includes primary ischemic injury and secondary reperfusion injury, which occur sequentially, acutely during cardiac arrest and resuscitation, and chronically in the post-resuscitation stage [24]. Transient global brain ischemia resulting from cardiac arrest in humans and animals causes neurodegeneration of neurons in the hippocampus, brain cortex, amygdala, basal ganglia, thalamus, dorsal and lateral septum, olfactory tubercle, primary olfactory cortex, entorhinal cortex, and brainstem [2,13,30,31,32,33,34,35,36]. The hippocampus is one of the brain regions most susceptible to ischemia after cardiac arrest in humans [37,38,39] and in animals [40,41,42,43]. Transient global brain ischemia causes selective neurodegeneration in the CA1 area of the hippocampus in humans and animals within 2–7 days after reperfusion [30,42]. Two years after ischemia in animals, in addition to neuronal loss in ischemia-sensitive areas, various stages of neuronal pathology were also observed in other areas [42]. Acute and chronic neuronal changes have been demonstrated in brain areas unrelated to primary ischemic pathology, i.e., hippocampal areas CA2, CA3, and CA4 [42]. In the hippocampus, activation of glial cells precedes neuronal loss and continues for a long time after an ischemic event in animals [44,45] and humans [46]. In the brains of humans and animals after ischemia, changes in the white matter combined with the proliferation of glial cells have been documented [42,44,45]. Autopsy of brains after experimental ischemia with a survival time of up to 2 years and in humans after ischemia showed severe hippocampal atrophy [42,47,48]. These neuropathological alterations have been clearly associated with progressive cognitive decline in humans [48,49,50] as well as in animals [51,52].
Brain ischemia caused by cardiac arrest increases the permeability of the blood–brain barrier in humans [53] as well as animals to cellular and non-cellular blood elements, i.e., platelets [54] and amyloids [55]. In the case of post-ischemic blood–brain barrier leakage, two facts deserve attention: the first is related to the passage of amyloid into the brain, and the second is the penetration of, among others, platelets containing huge amounts of soluble amyloid, which causes additional neurotoxic damage to the brain parenchyma [42,56]. Additionally, the permeability of the blood–brain barrier was influenced by oxidative stress, neuroinflammation [45,46,57], and the LRP1 and RAGE genes related to amyloid transport, described in detail later in the work. Soluble amyloid is delivered to the brain after cardiac arrest from the circulatory system [4,58] and additionally contributes to brain vessel vasoconstriction, amyloidosis, and cerebral amyloid angiopathy [49,50].
After brain ischemia, hippocampal atrophy was demonstrated with simultaneous neurodegenerative damage to the cortex of the temporal lobe [42,49,50]. Neuronal death after cerebral ischemia due to cardiac arrest may be dependent and independent of caspase. Moreover, neuronal death dependent on amyloid and modified tau protein has been demonstrated. Current preclinical and clinical evidence indicates that cardiac arrest causes chronic neuroinflammatory responses in the brain, which further exacerbate neurodegenerative changes [44,45,46]. Particularly extensive microglial activation and neurodegeneration in the CA1 area of the hippocampus in humans and animals are evident after cardiac arrest and lead to serious neurological sequelae [45,46,57].
3. Cognitive Deficits after Cardiac Arrest
The most sensitive brain regions to ischemic damage from cardiac arrest are the hippocampus, brain cortex, basal ganglia, thalamus, and amygdala [13,32,47]. These areas are closely related to cognitive domains (Figure 1) [13,51,52]. Cognitive functions depend on complex interactions between cortical and subcortical areas through various brain networks (Figure 1) [13]. Cognitive problems develop in 42–50% of patients who survive cardiac arrest up to several years after discharge from hospital [13,59]. Other studies have reported that in people who survived cardiac arrest, 50–100% of them experienced cognitive, mood, and functioning disorders [6,7,60]. Even among patients who returned to good neurological condition after discharge from the hospital, 29% experienced memory problems (short-term memory and spatial or contextual memory) and 43% experienced cognitive impairment [28,59].
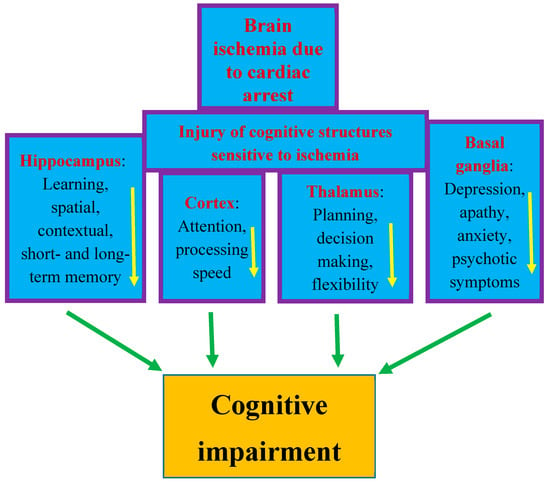
Figure 1. Structures susceptible to ischemic brain injury due to cardiac arrest and responsible for cognition. The figure shows damage to structures affecting memory, behavior, and cognitive impairment after cardiac arrest in an identical manner to that seen in Alzheimer’s disease. Arrows indicate decreased activity.
The post-ischemic hippocampus is believed to be the main structure underlying episodic memory impairment, which is the earliest and most visible clinical symptom preceding the development of dementia (Figure 1) [42,48,49,50,51]. Ischemia causes damage to the temporal cortex, which is the target area of the main axon output network from the hippocampus, so these areas are structurally and functionally related to each other and are important for learning and memory processes [13,49,50]. Thus, existing evidence indicates that cognitive and functional impairment after cardiac arrest is one of the important areas of concern for physicians [61,62,63,64,65,66,67]. The primary goal of research in the past was to improve survival after cardiac arrest. Currently, in clinical conditions, there is a search for methods that can improve neurological outcomes. Therefore, it is not surprising that research has moved beyond prognostication in the acute period following successful cardiopulmonary resuscitation to identifying mechanisms of long-term brain damage. Another currently promising area of research is the implementation of therapies that can reduce neuronal damage resulting from cerebral hypoxia and ischemia. It should be added that it is unlikely that survivors could be considered fully recovered immediately after being discharged from the hospital. Typically, sooner or later during long-term follow-up, they develop cognitive deficits, mainly related to memory and executive functions, or mild to severe impairment, including memory loss, deterioration of psychomotor, executive, and visuospatial functions, and emotional problems, including anxiety and depression [28,68,69,70]. The above-mentioned disorders affect the vast majority of patients after cardiac arrest—estimated to be as many as 88% [71]. The most frequently stated cognitive deficit is memory loss in patients who regain consciousness after cardiac arrest. It should be noted that memory with delayed recall and recognition is usually most affected, while isolated memory loss is rare. It can be summarized that the typical pattern of impairment after cardiac arrest includes deficits in memory, fine motor skills, and in executive functions [72]. According to the latest recommendations, before leaving the hospital, patients should be thoroughly assessed for short- and long-term memory deficits, cognitive and executive dysfunction, depression, and possible progression to dementia [62]. Overall, the prognosis for recovery of cognitive function to pre-arrest levels is uncertain. Despite uncertain prognoses, long-term rehabilitation has been shown to improve overall performance across a wide range of activities and should be pursued [73]. In most cases, patients are unable to return to daily activities within the first year after injury, and deficiencies are detected up to 8 years after cardiac arrest [74,75]. A study from Sweden, with an extremely long-term follow-up of 17 years after cardiac arrest, found a trend towards lower scores on cognitive tests and lower self-reported quality of life [71]. The authors concluded that survivors of cardiac arrest may experience permanent cognitive impairment, progressing to Alzheimer’s disease-type dementia [71,76]. It is suggested that the above-mentioned population is at increased risk of dementia due to the hypoxia occurring during cardiac arrest. It has been noted that the progression of cognitive decline following cardiac arrest in humans is characterized by greater memory impairment than executive dysfunction, making it similar to the prodromal form of Alzheimer’s disease dementia [76].
Cardiac arrest in humans and animals has been shown to significantly increase the risk of mild cognitive impairment, vascular cognitive impairment, and dementia, and dementia of Alzheimer’s disease type [46,51,52,77,78]. More than a quarter of patients experienced cognitive impairment four years after cardiac arrest [46,79]. Cohort studies have shown a high prevalence (54.4%) of long-term cognitive deficits and functional limitations in cardiac arrest survivors [46,80], even in those with apparently favorable neurological outcomes [46,81].
This entry is adapted from the peer-reviewed paper 10.3390/ijms25021291
This entry is offline, you can click here to edit this entry!
