1. Introduction
Forkhead box M1 (FOXM1) protein is a member of the forkhead box (FOX) transcription factor family that shares homology in the Winged Helix/
Forkhead DNA-binding domain [
1,
2,
3]. FOXM1 is highly expressed during normal embryogenesis and is extinguished in terminally differentiated cells [
4]. Homozygous deletion of
Foxm1 in mice is lethal; embryos die in utero between 13.5 and 17.5 days of gestation due to severe proliferation defects in multiple organs, including the heart, liver, and blood vessels [
5,
6]. Conditional deletion of
Foxm1 in various mice cell types inhibits cell proliferation [
4,
7,
8,
9,
10,
11,
12,
13], whereas the overexpression of
Foxm1 accelerates cell proliferation [
14,
15,
16,
17] and prevents age-related defects in cell cycle progression [
18]. FOXM1 also regulates the inflammatory response and intracellular metabolic processes [
19,
20,
21,
22,
23,
24,
25]. Consistent with the important role of FOXM1 in cell cycle progression, FOXM1 expression is increased during carcinogenesis [
26]. The human
FOXM1 gene is located on the chromosomal band 12p13 [
27], which is frequently amplified in different cancers, including prostate cancer [
28,
29], breast adenocarcinoma [
30], head and neck squamous cell carcinoma [
31], nasopharyngeal carcinoma [
32], and cervical squamous carcinoma [
33]. The increased level of FOXM1 in different types of cancer induces cancer progression, invasion, metastasis, and tumor-associated angiogenesis [
34,
35,
36]. The differential expression of FOXM1 in tumors compared to normal tissues makes it an attractive target for pharmacological inhibition [
26,
37]. Several FOXM1 inhibitors have been evaluated in pre-clinical studies. However, no compound has been advanced to clinical trials. Several natural products like honokiol, curcumin, genistein, solanum incanum extract, and diarylheptanoids were found to decrease the expression of FOXM1 and its target genes [
38,
39,
40,
41] or attenuate the
FOXM1 gene network [
42]. Cellular-based in vitro assays uncovered the FOXM1-inhibitory activities of thiazole antibiotics, including Siomycin A and thiostrepton [
43]. It was shown that the anti-FOXM1 activity of these compounds was executed through proteasome inhibition. Furthermore, pharmacological proteasome inhibitors, such as bortezomib and carfilzomib, inhibited FOXM1 activity to the same level [
44]. However, these FOXM1 inhibitors are not specific to FOXM1 and may carry severe off-targeted side effects [
45]. Therefore, there has been a movement to identify compounds that can specifically bind and inhibit FOXM1. Among them are RCM-1, STL427944, and STL001, representing the small molecules that inhibit FOXM1 nuclear translocation and induce its cytoplasmic degradation [
46,
47,
48,
49] (
Table 1). RCM-1 has shown excellent anti-tumor activity against different tumor cell lines and in mouse tumor xenografts without observed toxic side effects [
47,
50]. One mechanism by which RCM-1 inhibits FOXM1 is the disruption of protein–protein interactions between FOXM1 and β-catenin, a key receptor of the canonical Wnt signaling pathway. The inhibition of FOXM1–β-catenin interactions by RCM-1 results in the degradation of both proteins, leading to a robust anti-tumor effect [
47]. Other specific FOXM1 inhibitors, such as FDI-6 and XST-20, bind to the FOXM1 DNA-binding domain, which subsequently prevents FOXM1 interaction with DNA and decreases FOXM1 transcriptional activity without a decrease in protein level [
51,
52].
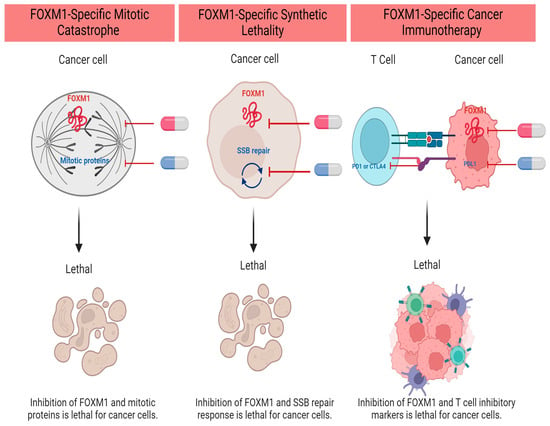
2. FOXM1 Inhibitors in Combination with Cytotoxic Chemotherapy
Cytotoxic chemotherapy is still the cornerstone for the management of most pediatric and adult malignancies. They are divided into categories based on their mechanism of action. Multiagent chemotherapy is frequently used to overcome cancer intrinsic resistance (Goldie–Coldman hypothesis). However, refractory and relapsed tumors are often encountered in clinical practice, with very few options left for salvage treatment. While chemotherapy resistance is often multifactorial, FOXM1 overexpression has been repeatedly observed in many resistant solid tumors [
73,
74]. Because FOXM1 has a critical role in DNA repair after cell exposure to DNA-damaging agents, its expression in resistant cells is a defensive mechanism to escape cell death. FOXM1 regulates the transcription of multiple DNA damage repair (DDR) proteins and enhances DNA single- and double-strand break repair [
73]. Furthermore, FOXM1 protein levels correlate with genomic instability and aneuploidy [
26,
75], and chromosomal instability is frequently linked to chemotherapy resistance and poor patient prognosis [
76,
77]. Collectively, FOXM1 is an attractive therapeutic target to be considered as an addition to chemotherapy to improve outcomes and prevent chemotherapy-resistant tumors (
Figure 1).
Figure 1. FOXM1 Inhibitor-Based Combination Therapies in Upfront Regimens. Created with
BioRender.com (accessed on 5 December 2023).
2.1. Combination with Alkylating Agents
a. Platinum analogs: Cisplatin and carboplatin work by forming DNA adducts that disrupt DNA structure, leading to irreparable damage and apoptosis initiation. Cisplatin-resistant ovarian and oral carcinoma cells express a higher level of FOXM1 [
78,
79]. Not surprisingly, FOXM1 overexpression correlates with the expression of multiple DNA damage response proteins such as BRCA2, XRCC1, and EXO1 [
74,
80], likely enhancing the efficiency of DNA repair and maintaining cell survival. FOXM1 also induces β-catenin expression, nuclear localization, and activation, promoting the epithelial-to-mesenchymal transition (EMT) and stem cell phenotype in ovarian cancer cells [
78]. Concurrent treatment with cisplatin and a FOXM1 inhibitor restores cisplatin’s anti-tumor activity. Combination treatment exhibits an enhanced proapoptotic activity in contrast to a single agent in ovarian cancer and oral squamous cell carcinoma xenografts [
78,
79].
b. Temozolomide (TMZ) is an alkylator that breaks DNA into double-strand DNA fragments. It is widely used for the treatment of high-grade gliomas. However, resistance to TMZ is usually inescapable and correlates with worse survival outcomes. Multiple studies demonstrated an increased FOXM1 level in TMZ-resistant cells [
61,
81,
82]. FOXM1 expression promotes DNA repair via the upregulation of RFC5 and Rad 51 proteins [
81,
82]. FOXM1 also upregulates the expression of the antiapoptotic protein Survivin, which has been linked to TMZ resistance [
61]. Collectively, concurrent treatment with thiostrepton or bortezomib and TMZ restores TMZ sensitivity and intensifies its apoptotic activity.
2.2. Combination with Topoisomerase II Inhibitors
Anthracyclines inhibit the topoisomerase II enzyme, intercalate between DNA bases, and cleave DNA into fragments. The accumulation of double-strand DNA breaks overwhelms the DNA repair response and drives the cells into apoptosis [
83]. In anthracycline-resistant breast cancer cells, FOXM1 is highly upregulated [
84]. FOXM1 overexpression is associated with an increase in DNA damage response proteins such as ATM and NBS1 [
85,
86]. It is also associated with the upregulation of antiapoptotic genes such as XIAP and Survivin [
87]. Ghandhariyoun et al. demonstrated that FOXM1 aptamer enhanced doxorubicin-induced apoptosis in breast cancer cells and mouse xenografts [
53]. Furthermore, thiostrepton increased doxorubicin accumulation in Jurkat cells due to the suppression of glutathione S-transferase pi (GSTpi) expression, a known culprit in multidrug resistance [
88].
2.3. Combination with Mitotic Spindle Inhibitors
a. Vinca alkaloids: Vincristine, vinblastine, and vinorelbine are tubulin inhibitors. They inhibit microtubule formation and lead to cell cycle arrest at mitosis. Donovan et al. demonstrated that the combination therapy of RCM-1 and VCR exhibited superior anti-tumor activity in contrast to single-agent therapy. The authors explored using a lower VCR dose to limit VCR-induced neuropathy and liver dysfunction while maintaining anti-tumor activity in rhabdomyosarcoma cell lines and mouse xenografts [
50]. Interestingly, RCM-1 can be injected intravenously using tumor-specific nanoparticles. Nanoparticle-based drug delivery enables more targeted and effective drug delivery and opens the door for anti-cancer combination therapies in a single infusion [
50]. Researchers previously showed that RCM1 treatment increases the duration of mitosis in tumor cells [
47], rationalizing the use of RCM1 with mitotic inhibitors to increase the efficacy of anti-cancer therapy.
b. Taxanes: Paclitaxel and docetaxel are similar to vinca alkaloids in that they inhibit tubulin and induce mitosis arrest. FOXM1 has an essential role in mitotic spindle formation, chromosome alignment, segregation, and daughter cell formation. Depletion of FOXM1 by thiostrepton (TST) downregulates the expression of the kinesin protein KIF20A, mediating mitotic spindle dysfunction and cellular senescence [
74]. In pancreatic cancer cells, TST inhibits the prohibin1 protein, decreasing the phosphorylated ERK1/2 level, and decreases the expression of the ABC drug transporter, fostering a higher intracellular anti-cancer drug concentration [
62]. Hence, TST synergizes with microtubule inhibitors, such as paclitaxel, to overcome drug resistance and induce mitotic catastrophe [
62,
63].
2.4. Combination with Antimetabolites
The antimetabolite family represents a large group of anti-cancer therapies, including folic acid antagonists and purine and pyrimidine analogs. They disturb DNA synthesis through the inhibition of key molecules in DNA’s structure.
a. Fluorouracil (5-FU) is a pyrimidine analog that blocks DNA synthesis through the suppression of the thymidylate synthase enzyme (TYMS) and the depletion of thymidine triphosphate. 5-FU is commonly used in adult solid tumors, including pancreatic and colon cancers. 5-FU-resistant colon cancer and cholangiocarcinoma cells exhibit high levels of FOXM1 and TYMS [
64,
65]. Moreover, FOXM1 binds directly into the TYMS promotor region and induces its expression [
64]. Hence, FOXM1 overexpression mediates 5-FU resistance because of the increase in drug targets [
64]. In addition, FOXM1 overexpression induces ABCC10 expression and increases drug efflux, promoting 5-FU resistance because of the decrease in the intracellular drug level [
89]. FOXM1 inhibitors, in combination with 5-FU, reduce colony formation, decrease cancer cell migration, and induce caspase-dependent apoptosis in colon and cholangiocarcinoma cancer cell lines [
64,
65].
b. Cytarabine is another pyrimidine analog used mainly in hematologic malignancies such as acute myeloid leukemia (AML). Cytarabine and anthracycline are the standard treatments for pediatric patients and medically fit adults with AML. Patients requiring more than one cycle of chemotherapy to achieve disease remission had worse survival outcomes [
66]. Chemotherapy resistance correlated with higher nuclear FOXM1 expression in post-treatment bone marrow samples [
66]. FOXM-1-overexpressed AML cells were resistant to standard chemotherapy in both in vitro and AML mouse models. Also, FOXM1 inhibition re-sensitizes resistant AML cells to cytarabine therapy. As a result, FOXM1 inhibitors can be studied concurrently or before standard AML chemotherapy to enhance treatment efficacy and restore drug sensitivity [
66]
This entry is adapted from the peer-reviewed paper 10.3390/cancers16040756