Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Neurosciences
Globally, thousands of people are affected by severe nerve injuries or neurodegenerative disorders. These conditions cannot always be cured because nerve tissue either does not regenerate or does so at a slow rate. Therefore, tissue engineering has emerged as a potential treatment approach. The combination of bioprinting, hydrogels, and drug delivery effectively addresses key issues in nerve tissue regeneration.
- bioprinting
- 3D printing
- tissue engineering
- nerve tissue engineering
- scaffolds
- drug delivery
1. Introduction
Severe injuries to the human nervous system can lead to profound consequences, primarily because it shows little to no capacity for self-regeneration. Annually, there are approximately 17,000 new cases of spinal cord injury (SCI), 80,000 cases of severe traumatic brain injury (TBI), and millions of cases of neurodegenerative diseases in the U.S. [1,2,3]. Additionally, 13 to 23 out of every 1 million individuals suffer from peripheral nerve injuries, often with poor prognoses [4]. Unfortunately, prevailing treatments focus primarily on symptom management rather than on tissue regeneration [5]. However, the emerging field of tissue engineering holds promise for developing therapies that can regenerate nerve tissue and restore its functionality.
The objective of tissue engineering is to create biological materials that can replace, restore, improve, or maintain the function of damaged tissues [6]. The most effective tissue engineering strategies use the interdisciplinary triad of tissue engineering: cells, scaffolds, and biochemical/physical signals [6]. Cell sources are autologous, allogeneic, or xenogeneic, ranging from stem cells or differentiated cells [6,7], while scaffolds, constructed from either natural or synthetic biomaterials, are typically fabricated via methods such as freeze-drying, electrospinning, decellularization, or bioprinting [8]. Biochemical signals refer to growth factors or pharmaceuticals [6], while physical signals involve mechanical loading. Efforts towards nerve regeneration present unique problems due to the structural and functional complexity of neural tissues. The limited capacity for self-regeneration creates a powerful impetus for alternative interventions to address nerve damage due to injury and degenerative diseases. Bioprinting, particularly with hydrogel-based bioinks, offers a promising solution to these challenges by enabling the creation of customized, three-dimensional, biomimetic structures. These structures can also be optimized for cell growth and natural tissue integration due to the versatility of polymeric hydrogels. Additionally, by employing hydrogels, drug delivery capabilities can be introduced into bioprinted scaffolds, thereby elevating the therapeutic potential of the system. By enabling the sustained release of neurotrophic factors or specific drugs, the system transitions from passively to actively promoting nerve regeneration and functional recovery. The combination of bioprinting, hydrogels, and drug delivery effectively addresses key issues in nerve tissue regeneration. This approach creates an optimal environment for nerve repair and regeneration and holds the potential to administer precise treatments to improve the regenerative process. Consequently, this strategy not only deals with the structural and functional intricacies of nerve regeneration, but also paves the way for more effective treatment methods for a wider range of neurological disorders.
2. Bioprinting
Three-dimensional bioprinting is an additive manufacturing process that uses cell-infused inks, called bioinks, to 3D-print complex tissue and/or organ resembling constructs [9,10]. In tissue engineering, the terms “3D printing” and “3D bioprinting” are often used interchangeably, but it is important to note their differences. Three-dimensional bioprinting uses bioinks that contain living cells and biologics to create tissues, while three-dimensional printing uses completely inert, non-living inks to create porous scaffolds to support cell attachment, proliferation, and differentiation [11]. In recent years, 3D bioprinting has rapidly grown in popularity due to its potential applications in tissue engineering and drug screening [12].
A typical bioprinting technique contains three main steps: pre-processing, processing, and post-processing [9]. Pre-processing consists of gathering imaging data, from computed tomography (CT), magnetic resonance imaging (MRI), ultrasound imaging, and/or optical microscopy [9]. The data are then transferred to computer-aided design (CAD) software to 3D-model the desired, patient-specific tissue, or create free-form models. The processing step of bioprinting includes bioink preparation and the printing process [9]. The most common bioprinting methods are described below and are summarized in Table 1 and the relative benefits and limitations are depicted in Figure 1. The post-processing phase of bioprinting includes maturing the printed tissue, typically by using a bioreactor to foster the growth and development of tissue in ideal environmental and mechanical conditions [13]. In theory, the tissue would then be ready for its desired application. In the context of neural tissue engineering, the bioprinting process is similar, with the exception that the use of bioreactors in the field has not yet been extensively studied [13].
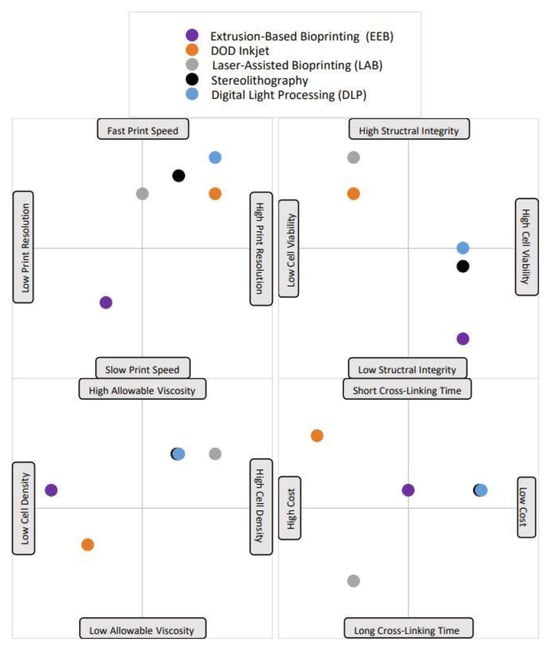
Figure 1. A visual representation of each bioprinting method’s advantages and disadvantages. Notably, each point is plotted based on the average value for that trait; some printing methods have very variable trait characteristics, but the standard value has been selected and is shown in the plot. For example, the printing speed of the droplet-based DOD inkjet method is highly variable and depends greatly on material viscosity. The majority of the time, low material viscosities are used with DOD, which leads to fast printing speeds, which is what is depicted here.
Table 1. The five (5) bioprinting process methods, a brief description of each, and a thorough comparison analysis of benefits and limitations. Eight criteria were selected to evaluate each process. The criteria are listed in order as follows: printing speed, cell resolution, structural integrity, post-printing cell viability, cross-linking time, allowable bioink viscosity, cell density, and cost. A ninth is present for methods with additional important criteria to consider.
| Method | Description | Advantages | Neutral | Disadvantages |
|---|---|---|---|---|
| Extrusion-Based Bioprinting (EBB) | Mechanical or pneumatic pressure creates continuous streams of bioink | Slow printing speeds | ||
| Low printing resolution | ||||
| High structural integrity | ||||
| Low cell viability | ||||
| Cross-linking time | ||||
| Must use low-viscosity bioinks (risk of nozzle clogging) | ||||
| High cell density allowable (although nozzle clogging is more likely) | ||||
| Average cost | ||||
| DOD Inkjet | Thermal, piezoelectric, or electrostatic mechanisms create droplets | Fast printing speeds | ||
| High printing resolution | ||||
| Low structural integrity | ||||
| High cell viability | ||||
| High cross-linking time | ||||
| Requires low-viscosity bioinks (risk of nozzle clogging) | ||||
| Low cell density (to reduce nozzle clogging) | ||||
| Low cost | ||||
| Laser-Assisted Bioprinting (LAB) | Laser pulses create areas of high pressure which force droplets onto a substrate | Medium printing speed | ||
| High printing resolution | ||||
| Low structural integrity | ||||
| Very high cell viability | ||||
| High cross-linking time | ||||
| Wide range of viscosities, low to very high (no nozzle, no nozzle clogging) |
||||
| Medium cell density (no nozzle clogging) | ||||
| High cost, not scalable | ||||
| Prone to metallic contaminants | ||||
| Stereolithography | Light polymerizes bioink in a layer-by-layer process | Medium printing speed | ||
| High printing resolution | ||||
| High structural integrity | ||||
| Medium cell viability | ||||
| Low cross-linking time | ||||
| Low to medium viscosity (no nozzle, no nozzle clogging) | ||||
| Medium cell density (no nozzle clogging) | ||||
| Medium cost | ||||
| UV radiation can cause cell and DNA damage | ||||
| Digital-Light Processing (DLP) | Light reflects off thousands of micromirrors and polymerizes whole layers at a time | Fast printing speed | ||
| Very high printing Resolution | ||||
| High structural Integrity | ||||
| Medium cell viability | ||||
| Low cross-linking time | ||||
| Low to medium viscosity (no nozzle, no nozzle clogging) | ||||
| Medium cell density (no nozzle clogging) | ||||
| Medium cost | ||||
| UV radiation can cause cell and DNA damage |
3. Bioink and Scaffold Considerations
3.1. Biological Properties
Biocompatibility is the most crucial characteristic for bioinks and biomaterial inks. To avoid adverse effects, these printing materials must be either biologically inert or immuno-compatible to ensure that no significant immune or inflammatory responses are triggered upon scaffold implantation [22]. Natural polymers, often considered biocompatible, are preferred for bioinks; however, numerous synthetic and composite polymers are also recognized for their biocompatibility [10].
Another vital feature of scaffolds is their biodegradability. Scaffolds are designed to gradually degrade, allowing native tissue to regenerate and replace them. Consequently, the degradation byproducts must be non-cytotoxic to avoid harming the cells [22]. Printed scaffolds also need to promote host cell adhesion. Ideally, they should enable host cells to infiltrate the scaffold, proliferate within it as it degrades, and ultimately substitute the damaged or lost tissue. To achieve this, scaffolds should possess a high degree of interconnected porosity [7] and emulate the extracellular matrix (ECM) of the target tissue [10].
3.2. Rheological Properties
Rheology is the study of the flow and deformation of a material under an external force [23]. One important rheological property is viscosity. High-viscosity printing materials can cause nozzle-clogging, but low-viscosity materials will not maintain their shape after printing. Furthermore, shear stress is another important factor related to viscosity. High shear stresses can be caused by printing pressure, nozzle diameter, and the viscosity of the bioink [23], and have been shown to decrease post-printing cell viabilities. Additionally, high-viscosity bioinks themselves can impose high shear stresses on cells [14]. Shear thinning can occur when the viscosity of a material decreases as the shear rate increases, and is another challenge to consider [23]. Overall, rheological properties affect which bioprinting method can be used, as well as the resulting cell viability, printing resolution, and integrity of the printed construct.
3.3. Electrical Properties
One material property unique to neural tissue engineering is electrical conductivity. Electrically conductive scaffolds are necessary because they mimic native neural tissue ECM and can support axon regeneration in vivo [17]. A study by Heo et al. added intrinsically conductive polymers to a GelMA bioink, which enhanced its electrochemical properties and significantly improved the differentiation of dorsal root ganglion cells under electrical stimulation [24]. Other studies have added polypyrrole in their scaffolds to improve their conductive and electrical properties. These studies have determined that enhanced electrical properties in scaffolds improve neurite outgrowth, as well as axonal remyelination and regeneration in vitro [17].
3.4. Mechanical Properties
The mechanical properties of scaffolds should closely mimic those of the native neural tissue. The elastic moduli of scaffolds have been shown to influence cell signaling and affect adhesion, differentiation, and proliferation of neurons [10,17]. Protocols for differentiating stem cells into neurons require hydrogels with elastic moduli between 1 and 10 kPa. Hydrogels with higher stiffness may inadvertently cause stem cells to differentiate into myogenic or osteogenic cells [17]. The mechanical properties of various neural tissues vary: brain tissue typically exhibits a stiffness of about 0.5 kPa, peripheral nerve tissue has an ideal stiffness around 450 kPa, and spinal cord tissue has a stiffness that can range between 200 and 600 kPa [10,17]. Furthermore, creating scaffolds with similar elastic moduli should incorporate other factors to ensure differentiation into neural tissue. These scaffolds need to have similar tensile, compressive, and shear strengths as the native tissue. Thus, it is important to view these properties dynamically, in relation to the duration of implantation, rather than as fixed characteristics [10].
3.5. Bioink Cell Considerations
Bioinks can be defined by the incorporation of cells, and possibly biologically active components and biomaterials, into a mixture suitable for 3D bioprinting. Bioinks should not be confused with biomaterial inks, which are biomaterials suitable for 3D printing and are not cell-laden. However, biomaterial inks can be seeded with cells once printed [25]. Bioinks used in neural tissue engineering commonly include human-induced pluripotent stem cell (hiPSC)-derived neural progenitor cells (NPCs) and other neuronal stem cells (NSCs). The encapsulation of cells in bioinks poses many challenges. High cell concentrations in bioinks can cause nozzle-clogging, but low cell concentrations do not mimic native tissue, and make it less feasible to scale the scaffold to human dimensions [9]. Furthermore, it is important to not introduce cytotoxic agents during the 3D printing process [10] or sterilization processes. Chemical cross-linking agents such as photoinitiators could be cytotoxic, as could the UV radiation commonly used to solidify the gels [10]. Lastly, many sterilization processes would likely cause cell damage, so any future clinical applications of cell-laden scaffolds need to utilize appropriate and non-cytotoxic sterilization methods.
3.6. Hydrogels as Biomaterial Inks and Bioinks
Hydrogels are typically the top candidates for biomaterial inks. Hydrogels are 3D structures made from cross-linked hydrophilic polymers and can swell and absorb up to one thousand times their dry weight with water [22]. Hydrogels are ideal for 3D printing, as they can be printed in a liquid, low-viscosity state, and can be chemically or physically cross-linked to solidify and form a stable 3D structure. Hydrogels are particularly useful as they can encapsulate cells and small molecules, such as drugs and some proteins [5]. They also have many tunable properties, such as biocompatibility, biodegradation, mechanical strength, viscosity, porosity electrical conductivity, responsiveness to environmental stimuli, etc., and can be modified to have ideal properties. Furthermore, hydrogels can mimic the structure of the native ECM [7,23]. Lastly, high-porosity hydrogels provide a suitable environment for nutrient exchange, allowing for cell adhesion, proliferation, and migration [7,23].
This entry is adapted from the peer-reviewed paper 10.3390/biophysica4010004
This entry is offline, you can click here to edit this entry!
