Until recently, the clinical staging relied on clinical evaluation, preferably by an experienced examiner
[1]. Conventional procedures, such as cystoscopy, proctoscopy, intravenous urography, and X-ray of the lungs, were performed
[1]. Examination under anaesthesia was recommended, especially when the examination was difficult, or there was uncertainty regarding the involvement of the vagina, parametrium, or pelvic wall
[2]. However, physical examination alone is known to have low accuracy for assessing tumour size and parametrial infiltration. Historically, Innocenti et al. reported sensitivities of clinical evaluation and transrectal ultrasound for diagnosing parametrial involvement, using surgico-pathology as the reference standard, of 52% and 78%, respectively
[3]. Twenty years ago, the multicentric clinical trial of the American College of Radiology Imaging Network/Gynecologic Oncology Group (ACRIN/GOG183) enrolled 208 patients (from 25 centres, with invasive cervical cancer proved by biopsy results) who underwent pelvic magnetic resonance imaging (MRI) and contrast-enhanced computed tomography (CT) before definitive radical hysterectomy
[4]. Correlation between maximum histopathologic tumour size and tumour size from clinical assessment (rs = 0.37, low correlation), CT (rs = 0.45, moderate correlation), and MRI (rs = 0.54, moderate correlation) were reported with the highest figures for MRI
[4]. It is obvious that clinical assessment alone is insufficient to accurately assess the size of smaller tumours that do not cause cervical enlargement. In the same ACRIN/GOG 183 study, MRI yielded higher sensitivity (53%) than clinical assessment (29%) for diagnosing parametrial invasion
[5]. A systematic review including 3254 patients also found that MRI had much higher sensitivity than clinical examination for diagnosing parametrial invasion (sensitivity: 84% vs. 40%) and locally advanced disease (79 vs. 53%)
[6]. Clinical understaging can be caused by the inability to detect incipient parametrial invasion by clinical examination, especially ventrally and/or positive (metastatic) lymph nodes, while clinical overstaging can be due to subjective clinical assessments falsely interpreted as parametrial spread. Although findings from modern imaging examinations (i.e., ultrasound, MRI, CT, and PET-CT) were not considered for staging purposes in the guidelines until 2018, they had already largely replaced staging results based on conventional procedures in many gynaecologic oncology centres in high-income countries. A multicentric clinical trial by the American College of Radiology Imaging Network/Gynecologic Oncology Group (ACRIN/GOG183) from 2005 showed only sporadic use of conventional procedures recommended for cervical cancer staging
[5][7]. Cystoscopy was performed in 8.1%, sigmoidoscopy or proctoscopy in 8.6%, intravenous urography in 1%, and examination under anaesthesia in 27%
[5][7]. Similar trends were reported in the European Society of Gynaecological Oncology (ESGO) survey published in 2018; cystoscopy was used during preoperative workup in 17%, rectoscopy in 10%, and examination under anaesthesia in 26%
[8]. Therefore, in 2018, the initiative to update the guidelines was undertaken under the joint umbrella of ESGO, the European Society for Radiotherapy and Oncology (ESTRO), and the European Society for Pathology (ESP) and new guidelines for the staging, treatment, and follow-up of cervical cancer patients were established, implementing imaging into the staging and treatment decision-making process
[9]. Of note, the updated recommendations after five years (2023) are unchanged in terms of recommended imaging modalities for local, nodal, and distant staging of this disease
[10].
2. Local (Pelvic) Workup for Different Stages
The role of modern imaging in local staging is to delineate the cervical tumour to determine if fertility-sparing surgery can be offered, tailor the radicality of parametrial resection based on the minimum thickness of uninvolved cervical stroma, and to assess parametrial infiltration and tumour invasion into the pelvic side wall or adjacent organs (bladder, rectum). Following the revised FIGO 2018 staging system for cervical cancer
[11][12], imaging methods include ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), combined PET-CT or PET-MRI, etc., based on local resources
[13]. In the ESGO survey published in 2018, CT, PET-CT, MRI, and ultrasound were frequently used in the pre-treatment diagnostic workup. The survey showed that in early-stage disease, MRI was the most frequently used imaging method (74%), but more than half of the respondents used CT (54%), and a minority preferred PET-CT (25%). Pelvic ultrasound was reportedly considered in 23%
[8]. Real-life data on gynecologic oncologists’ preferred primary staging modality and their diagnostic performance in early-stage cervical cancer was published in the prospective, international SENTIX study
[14]. Each participating site was instructed to choose their preferred method based on their routine clinical practice. Among 690 prospectively enrolled patients with early-stage cervical cancer, 46.7% and 43.2% of patients underwent MRI and pelvic ultrasound, respectively, whereas 10.1% underwent both modalities. Pelvic MRI and ultrasound yielded similar diagnostic performance for predicting histological tumour size, parametrial involvement, and macrometastatic nodal involvement. CT is a well-established imaging method being widely used in cancer staging. Improvements in CT technologies using helical and multi-detector CT (MDCT) yield higher spatial resolution with shorter acquisition times, albeit with reported slightly higher radiation exposure
[15]. However, CT is still inferior to MRI in assessing tumour size and local tumour extension due to its lower soft-tissue contrast resolution, even when using contrast-enhanced (CE) CT
[4][5][16][17][18]. Iodinated contrast agents are routinely used for CT examinations to visualise contrast-enhancing neoplastic lesions or metastases and diagnose various non-malignant conditions, e.g., infectious- and vascular disease (
Figure 1)
[4][16]. PET-CT provides a unique combination of anatomic information provided by CT and tissue-specific metabolic characteristics provided by PET using the glucose analogue (18)F-fluorodeoxyglucose (FDG). The fused images acquired during a single examination facilitate localising malignant lesions, typically exhibiting increased FDG-avidity and depiction of physiologic FDG uptake in non-malignant tissue. However, PET-CT is not optimal for local staging due to the low soft-tissue contrast on CT and the low spatial resolution of PET. Furthermore, the partial volume effect from FDG-avid urine in the bladder (physiologic renal FDG excretion) may preclude an accurate definition of the tumour volume or parametrial invasion (
Figure 1)
[19]. PET-CT involves slightly higher radiation exposure than diagnostic CT alone
[20]. MRI thoroughly assesses the pelvic anatomy with a wide field of view and no radiation risk. MRI has for years been considered the modality of choice for detecting local tumour spread
[21] and has been shown to yield high interobserver reproducibility for tumour size measurements with high concordance between maximum primary tumour size from MRI and from hysterectomy specimens
[22]. However, MRI is relatively expensive and time-consuming and may be contraindicated in some patients (e.g., in the presence of MRI-incompatible implants). Furthermore, limitations in access to MRI scanners are particularly common in low-income countries. As for all imaging modalities, their diagnostic accuracy depends on the radiologist’s experience in gynaecologic oncologic imaging. The MRI protocol, traditionally based on morphological sequences, has recently been supplemented by functional sequences, including diffusion-weighted imaging (DWI) and dynamic contrast-enhanced (DCE) MRI (
Figure 1)
[23][24]. DWI depicts the free water motion of the tissue. The free water movement is restricted in malignant lesions, normally being highly cellular. The tumour appears hyperintense on high b-value (e.g., b = 1000 s/mm
2) images and correspondingly hypointense on the apparent diffusion coefficient (ADC) maps. The ADC map depicts true restricted diffusion and allows measurements of tumour ADC value. DWI combined with conventional MRI sequences enable assessments of both morphologic and physiologic features in a single examination. In DCE-MRI, dynamic image acquisition is accomplished after the administration of an intravenous bolus of gadolinium-based contrast agent. Typically, cervical tumours enhance rapidly, followed by an early washout of contrast. In the early arterial phase (30 sec post-contrast), the tumour is hyperintense. In contrast, in the late venous phase (2 min post-contrast), it is hypointense relative to the more gradually enhancing normal cervical epithelium and stroma
[17][23]. Using a contrast agent could increase the reader’s confidence in identifying stromal and parametrial invasion. On the other hand, no significant improvement in staging accuracy has been demonstrated, and therefore, its use is not considered essential
[25]. The addition of DWI to T2-weighted MRI demonstrated a promising role in improving the detection of parametrial invasion and increasing reader confidence
[26][27], allowing better tumour delineation for less-experienced radiologists. Importantly, the measured maximum tumour dimensions are reportedly virtually identical based on DWI and conventional series. Functional magnetic resonance, including DWI and DCE-MRI imaging, have also been recently addressed and studied as a tool supplementing conventional MRI in brachytherapy settings for patients with locally advanced cervical cancer. Their complementary use resulted in lower interobserver variability in target delineation (Gross tumour volume)
[28]. Nevertheless, validation through robust prospective data, before extensive adoption of DWI and DCE-MRI in cervical cancer, is essential. Particular attention must be paid to the use of uniform protocols, standardised nomenclature and correlation of imaging findings with histopathology
[29][30]. PET-MRI integration has not yet been shown to significantly improve local staging performance compared to MRI alone
[31]. Examination from the renal hila to the pubic symphysis is recommended to assess the presence of hydronephrosis in case of pelvic wall and lymph node infiltration (see below)
[24].
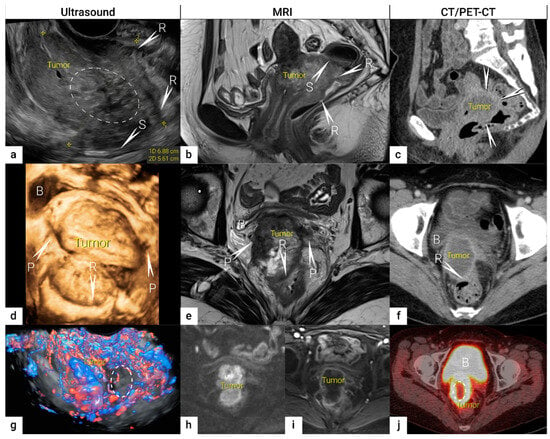
Figure 1. Bulky cervical squamous cell carcinoma in a 48-year-old woman with FIGO stage IVA (T4 N1 M0).Upper row: a 7 cm large (maximum diameter), mostly necrotic tumour depicted in the sagittal plane by (a) transrectal US (hypoechogenic tumour marked with dotted line having central irregular necrotic cystic areas (ellipse); (b) T2-weighted MRI depicting a hyperintense cervical lesion and contrast-enhanced (CE) CT (c) depicting a hypodense tumour. The infiltration of the rectal wall (R) and sigmoid colon (S) are marked with arrows and single letters of corresponding adjacent organ infiltration. Middle row: the same tumour depicted in the transverse plane by transrectal three-dimensional ultrasound (d); T2-weighted MRI (e) and CE CT (f); exhibits infiltration of lateral parametria (P) and rectum (R) marked with arrows and single letters of corresponding adjacent organ infiltration. Lower row: three-dimensional colour Doppler US (g) depicts high perfusion within the same tumour except in the central necrotic part (ellipse). Paraaxial DWI (h) (high b-value image: b = 1000) depicts restricted diffusion within the tumour and contrast-enhanced MRI (DCE-MRI) (i) enhancement rim in the periphery of the lesion with central necrosis. Axial FDG- PET-CT (j) depicts FDG-avid tumour except for the central necrotic part (ellipse). B, bladder; FDG, fluorodeoxyglucose; FIGO, the International Federation of Gynaecology and Obstetrics; CT, computed tomography; MRI, magnetic resonance imaging; P, lateral parametria; PET-CT, positron emission tomography fused with computed tomography; R, rectum; S, sigmoid colon.
The diagnostic potential of ultrasound is likely to have been underestimated in gynaecologic oncology until recently. In cervical cancer, ultrasound was formerly reserved for the screening of hydronephrosis. The reported limitations of ultrasound were low-contrast resolution, which may have limited the differentiation of the tumour from the adjacent tissue, small field of view disallowing the evaluation of the pelvic side wall, dependence on operator skill, subjectivity when interpreting the image, and challenging technical storage and retrieval of high-quality images on demand. However, ultrasound has undergone major technological developments over the past decades, especially the development of endovaginal high-resolution probes with a wide field of view, allowing the depiction of detailed pelvic anatomy comparable to that from MRI (Figure 1).
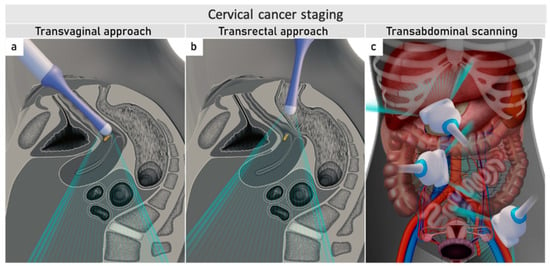
Ultrasound can be performed by gynaecological oncologists who benefit from the meticulous knowledge of the disease. The same endoluminal probe can be introduced transvaginally or transrectally. The transrectal approach, performed without any patient preparation, such as enema or fasting, is preferred for cervical cancer due to the diminished risk of bleeding from the tumour. Additionally, the transrectal approach allows a better acoustic setting to show the distal part of the cervix
[32]. The combination of transvaginal/transrectal and transabdominal ultrasound allows a complete assessment of the abdomen and pelvis for abdominal staging (
Figure 2)
[33].
Figure 2. Ultrasound for cervical cancer staging.Transvaginally inserted probe (a). Transrectally inserted probe (b). Transabdominal scanning (c).
Nowadays, expert sonographers dedicated to gynaecologic oncology scanning can perform local staging by evaluating the maximum tumour size, depth of stromal invasion, infiltration of pericervical fascia and parametrial involvement up to the pelvic side wall, including the assessment of iliac vessels, pelvic muscles and nerves, and the depth of bladder and rectal wall infiltration
[34][35][36][37][38]. Moreover, the dynamic aspect of an ultrasound scan can be applied to reliably exclude the infiltration of adjacent organs (bladder and rectosigmoid colon)
[39][40]. In addition to two-dimensional (2D) ultrasound, three-dimensional (3D) ultrasound obtains the third (coronal) plane, similar to MRI. It enables easy storage of measured volumes and their retrieval for second readings, planning radiotherapy, and evaluating the treatment effect (
Figure 3). Moreover, Doppler ultrasound allows highly accurate, non-invasive, in vivo assessment of vascular features reflecting tumour angiogenesis, which has been shown to predict clinical response to neoadjuvant or definitive chemoradiation in patients with locally advanced cervical cancer
[41][42]. The transabdominal ultrasound not only evaluates hydronephrosis
[43] but may also detect abdominal intraparenchymatous metastases, lymph node metastases, and peritoneal spread during one examination (see below)
[33]. The ultrasound examination is well tolerated by patients and does not require any patient preparation, such as fasting or contrast agent application. Contrast-enhanced ultrasound is not established in cervical cancer staging
[44][45]. In addition, ultrasound is widely available and cheaper than MRI and does not have any known contraindications (
Table 1 Comparison of Different Imaging Methods for Application in Gynecologic Oncology).
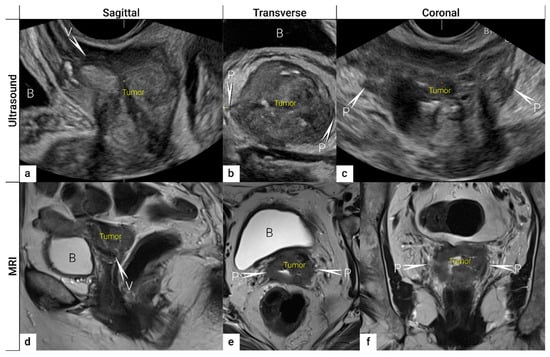
Figure 3. Three imaging planes of three-dimensional (3D) ultrasound and MRI. Cervical squamous cell carcinoma in a 57-year-old woman with FIGO stage IIIC1 (T2b N1 M0). Sagittal (a), transverse (b), and coronal plane (c) on 3D ultrasound depict a large tumour of 5 cm (max diameter) as a hypoechogenic mass. T2-weighted MRI images in sagittal (d), transverse (e), and coronal (f) planes visualise the tumour as a slightly hyperintense cervical mass. Both imaging methods showed in the sagittal view an infiltration of the ventral vaginal wall (V) and in the transverse and coronal plane a bilateral invasion of lateral parametria (P) marked with arrows and single letters of corresponding adjacent soft tissue infiltration. B, bladder; MRI, magnetic resonance imaging; V, vagina.
Table 1. Comparison of Different Imaging Methods for Application in Gynecologic Oncology.
The introduction of ultrasound into cervical cancer staging depends on the availability of a trained sonographer. Whereas MRI could acquire images without needing an on-site MRI specialist with subsequent readings by an experienced radiologist. Another limitation may be the lack of routine standardised storage of ultrasound images, which makes it impossible to present ultrasound images at multidisciplinary meetings. ESGO, the International Society of Ultrasound in Obstetrics and Gynecology (ISUOG), FIGO and other scientific societies recognise the role of ultrasound in gynecologic oncology, particularly for cervical cancer in low-resource countries where the incidence is highest and availability of imaging is limited. ESGO and ISUOG have recently introduced training in gynaecological-oncology imaging as part of their curricula. An experienced sonographer should document the extent of the disease using a predefined checklist and high-quality dynamic and 3D images with easy on-demand retrieval. There are no acoustic limitations to pelvic scanning, such as obesity or intestinal gas, which may limit abdominal retroperitoneal staging in some patients (Table 1).
Acknowledging some of the advantages of ultrasound over MRI (
Table 1 Comparison of Different Imaging Methods for Application in Gynecologic Oncology), the next step before implementing ultrasound as a possible first-choice procedure for pelvic staging is to demonstrate its comparable diagnostic performance with that of MRI, in the same patient cohort. The diagnostic performance of imaging is ideally assessed using histopathology as the reference standard. Full-section tumour specimens are easily available in early-stage cervical cancer undergoing definitive surgical treatment. Thus, evidence for the role of ultrasound in comparison to state-of-the-art imaging modality (MRI) in local staging of early-stage cervical cancer can be retrieved from recent European multicentric prospective trials comparing both imaging modalities in the same cohort, the results of which are presented in the text below
[36]. Whereas in patients with locally advanced cervical cancer who are subjected to definitive chemoradiation, histological confirmation of local infiltration is missing. In lack of a true reference standard, some authors have tried to compare ultrasound to MRI using MRI as a reference standard
[32], although studies have reported MRI to yield similar or lower accuracy than ultrasound for staging in early-stage cervical cancer
[36]. Although MRI is significantly better than CT (
p = 0.047) for detecting parametrial invasion with reported high specificity (77–80%) and negative predictive value (83–87%), the reported sensitivity (40–57%) and positive predictive value (32–39%) of MRI for detecting incipient parametrial spread are still low
[46]. Another important aspect of the implementation of the imaging method of choice in primary work-up is to prove their inter-observer reproducibility
[47][48]. A multicentric trial comparing the inter-observer agreement of transvaginal ultrasound and MRI in the assessment of local tumour extension in cervical cancer patients in relation to observer experience showed that the inter-observer agreement was moderate for ultrasound (κ values 0.41–0.60), and moderate-substantial (κ values 0.61–0.80), for MRI. The experience of the ultrasound examiners was associated with inter-observer agreement only for parametrial invasion
[47]. The results emphasise the importance of systematic training of the sonographers performing cervical cancer staging
[47]. Interestingly, no difference in agreement for staging parameters among radiologists with different levels of pelvic MRI experience was reported in a recent cervical cancer MRI patient cohort (n = 416)
[49].
Data concerning the local tumour staging using the recommended imaging methods is given in the following steps, from tumour detection and delineation to assessment of its extent in the surrounding organs:
2.1. Tumour Detection
The first step in local staging by ultrasound or MRI is the identification of cervical cancer tissue, which is assessed in relation to the surrounding cervical stroma. On ultrasound, a squamous cell cancer is characterised by a hypoechogenic, richly vascularised tumour, while adenocarcinoma is more often an iso- or hyperechogenic, highly perfused lesion contrasting with healthy residual cervical stroma (
Figure 4)
[50]. On T2-weighted MRI, cervical cancer is typically an iso- or hyperintense mass regardless of histopathologic type, and when small, is surrounded by a hypointense normal cervical stroma (
Figure 4)
[51]. Tumour hyperintensity on the high b-value DWI with corresponding low intensity on the ADC map represents true restricted diffusion in the lesion. Importantly, the inclusion of DWI in addition to T2-weighted imaging in cervical cancer has been shown to improve tumour detection and reader confidence and yield better diagnostic accuracies for predicting parametrial invasion
[27][52]. Promising results on early-stage tumour detection by ultrasound versus MRI based on single-unit studies
[34][35] have been reported by a European multicentre trial including 189 patients operated for early-stage cervical cancer
[36]. The transvaginal or transrectal ultrasound/MRI (based on T2-weighted and T1-weighted series without DWI) yielded accuracies (sensitivities) [specificities] of 96%/86%,
p < 0.001 (90%/67%,
p = 0.008) [97%/89%,
p = 0.005] for tumour detection. This research found an excellent agreement between ultrasound and final histology for detecting tumours (kappa value 0.84), while the agreement between MRI and final histology was only moderate (0.52).
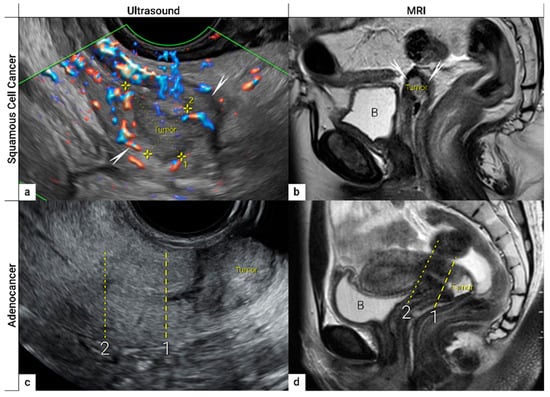
Figure 4. Imaging of cervical squamous cell carcinoma and adenocarcinoma. Upper row: cervical squamous cell cancer in the remaining cervix after previous supravaginal hysterectomy (due to uterine prolapse) in a 59-year-old woman with clinical FIGO stage IB1 (T1b1 N0 M0), depicted in the sagittal plane by transrectal ultrasound (a) as a 2 cm large (maximum diameter) hypoechoic tumour marked with callipers and surrounded by a rim of a non-involved hyperechoic stroma (arrows), sagittal T2-weighted MRI (b) depicting a hyperintense cervical lesion surrounded by hypointense normal cervical stroma (arrows) in the same patient. Lower row: adenocarcinoma stage T1b1 N0 M0 located on the exocervix in a 28-year-old woman before fertility-sparing surgery and depicted in the sagittal plane by transrectal ultrasound (c) as a 3 cm large hyperechogenic tumour and by T2 weighted MRI (d) as a hyperintense tumour. 1- cranial tumour margin; 2- internal os; distance between the dotted and dashed line corresponds to the cranial tumour-free margin. B, urinary bladder.
2.2. Tumour Delineation within Cervix (Tumour Size, Depth of Stromal Invasion, Minimum of Uninvolved Stroma, and Cranial Tumour-Free Margin)
The next step is to delineate tumour borders within the cervix to assess important prognostic parameters (tumour size, depth of stromal invasion, minimum of uninvolved stroma, and cranial tumour-free margin and others), which guide the treatment options and enable individualised management, including fertility-sparing treatment (FST). To assess eligibility for FST, the joint ESGO-ESTRO-ESP cervical cancer guidelines recommend ultrasound and MRI as the imaging tests of choice to measure remaining cervical length (after cone biopsy), uninvolved cranial tumour-free margin, and residual tumour size (
Figure 4)
[9]. FST is only feasible in squamous cell cancer or HPV-associated adenocarcinoma with a largest diameter of less than or equal to 2 cm
[9]. A multicentric European trial focused on the tumour detection rate after cone biopsy, comparing the diagnostic performance of ultrasound and MRI (without DWI)
[36]. The residual tumour detection rate after cone biopsy was not significantly different from tumour detection rate after cervical biopsy alone if assessed on ultrasound (sensitivity:
p = 1.0; specificity:
p = 0.23) or MRI (sensitivity:
p = 0.78; specificity:
p = 0.25)
[36]. The study reported good agreement between ultrasound and MRI in classifying small tumours less than 2 cm (kappa values 0.78 and 0.71, respectively). For FST planning, the most important parameter to be evaluated is the actual tumour-free distance between the cranial tumour margin and the internal os (
Figure 4). A recent meta-analysis published by Xiao et al. in 2020 on the diagnostic performance of MRI in evaluating the distance between the tumour and the internal os on six studies (454 patients) showed a pooled sensitivity and specificity of 87 and 91%, respectively
[53]. A meta-analysis published by Woo et al. in 2020 analysed five MRI studies and showed a pooled sensitivity of 84% and specificity of 96% in detecting internal os involvement
[54]. A multi-institutional prospective study compared the accuracy of ultrasound and MRI for tumour detection, tumour size measurements, parametrial-, uterine corpus- and vaginal fornix involvement and prediction of FIGO/T-status (from TNM system), reporting no significant difference between the two imaging methods
[55]. Regarding assessment of cervical internal os invasion and uterine corpus infiltration as a negative prognostic factor, the accuracy for ultrasound and MRI was 94% and 86%, respectively (
p = 0.3)
[55]. On top of that, ultrasound can also be used to guide the surgeon intraoperatively during FST to determine the optimal level of excision in order to preserve the maximum length of tumour-free cervix for a future pregnancy
[56]. In the pilot study, the cranial tumour-free margin was marked intraoperatively under ultrasound guidance with a non-absorbable suture
[56]. The pathology report showed a mean distance between the stitch and the cranial tumour margin of 1.5 mm (SD 1.16, range 0.09–5 mm)
[56]. In cases in which neoadjuvant chemotherapy (NACT) precedes FST with the aim to achieve partial or complete tumour response with sufficient cranial tumour-free margin, MRI or ultrasound in experienced hands can also be used to assess the treatment response
[57]. In a single-unit prospective study comparing MRI and ultrasound after NACT in 42 patients using pathological results as a reference, the agreement between measurements obtained by MRI (without DWI) and histology was not found statistically significant (intraclass correlation coefficient; 0.344; 95% CI: −0.013 to 0.610;
p = 0.059), while agreement between transrectal ultrasound and histology reached statistical significance (intraclass correlation coefficient; 0.795; 95% CI: 0.569–0.902;
p < 0.001)
[57]. Individualised planning of care is not only limited to FST but also to assess the radicality of hysterectomy or primary chemoradiotherapy, depending on the tumour size and depth of stromal invasion. Tumour size >4 cm and deep stromal invasion are indicative of a worse prognosis; thus, they are used in many centres as an indication for primary chemoradiation instead of primary surgery. The multicentric European trial results demonstrated almost perfect agreement between ultrasound and histology in the assessment of such bulky tumours (>4 cm) and deep stromal invasion (kappa values 0.82 and 0.81, respectively)
[36]. The agreement between MRI (without DWI) and histology was substantial for the classification of bulky tumours (>4 cm) and detection of deep stromal invasion (kappa values 0.76 and 0.77, respectively)
[36]. Recently, the measurement of the distance between tumour and parametria (tumour-free distance or a minimum uninvolved stroma) has been recommended as it better correlates with the risk of extrauterine extension and nodal metastasis rate than the tumour size or depth of stromal invasion, which does not consider the size of the cervix and the tumour location within the cervix
[58][59]. It is measured as the remaining uninvolved fibromuscular stroma between the tumour and pericervical fascia at the point where the ventral, lateral, and dorsal parametria are attached to the cervix. The cut-off values for tumour-free distance associated with clinical outcomes ranged between 2.5 and 3.5 mm but without prospective validation
[58][59]. Looking at the available evidence, pre-surgical MRI showed a sensitivity of 88% and a specificity of 75% in the assessment of tumour-free distance
[60].
2.3. Extrauterine Extension (Vagina, Parametria, Pelvic Side Wall, Hydronephrosis and Others)
The third step is the assessment of extrauterine extension. The vaginal extension is routinely assessed during physical examination. The estimation of vaginal fornix using imaging can be difficult especially in large tumours stretching the vaginal fornix. An optional, useful tool to better assess the vaginal extension of the tumours is represented by vaginal opacification with gel, especially when the region of interest is represented by the posterior vaginal fornix. On the other hand, the role of imaging is critical to assess pericervical fascia and parametrial involvement, including pelvic side wall invasion. Visualisation of intact pericervical fascia surrounding the cervix as a hyperechogenic line on ultrasound or a hypointense line on MRI excludes infiltration of parametria with specificity and a negative predictive value of 98–100%
[34][61][62]. In addition, the dynamic aspect of ultrasound examination helps to establish parametrial status. Especially in situations with limited visibility, the exertion of sliding of the tumour against the surrounding tissue planes is a sign of intact parametria. A multicentric European trial of early-stage cervical cancer of transvaginal or transrectal ultrasound/MRI (based on T2-weighted and T1-weighted series without DWI) yielded accuracies (sensitivities) [specificities] of 97%/90%,
p = 0.001 (77%/69%,
p = 0.56) [98%/92%,
p < 0.001] in the assessment of parametrial invasion
[36]. A recent meta-analysis published by Alcázar et al. in 2020 reported similar diagnostic performance for detecting parametrial invasion in cervical cancer by ultrasound/MRI, with pooled sensitivities and specificities of 78%/68% and 96%/91%, respectively
[63]. No statistical differences were found when comparing both methods (
p = 0.548)
[63]. These data were confirmed by another meta-analysis published in 2020 by Woo et al. and showed that ultrasound has a comparable level of diagnostic performance to MRI in assessing parametrial invasion (pooled sensitivities and specificities of 67%/71% and 94%/91%, respectively)
[54]. Apart from establishing the involvement of parametria, the precise localisation of infiltration (ventral right/left, lateral right/left, dorsal right/left) represents an added benefit for radiotherapy planning (
Figure 1 and
Figure 3)
[32]. A narrated video by Moro et al. guided the reader through the methodology of assessing the structures surrounding the cervix and vagina (specifically the parametrium)
[64]. Chiappa et al. compared the agreement between 2D and 3D ultrasound to MRI results as a reference in assessing parametrial invasion and showed the best agreement in the assessment of ventral parametria (90% and 62.5%), followed by the right lateral parametrium (72% and 81%), left lateral parametrium (69% and 70%), and dorsal parametria (58.5% and 52%)
[32]. Based on the study results, 2D- and 3D-ultrasound showed similar moderate agreement with MRI. In addition to the location of infiltrated parametria, the degree of parametrial invasion can also be assessed on ultrasound or MRI using a standardised grading system, which is crucial for the treatment choice
[33]. The features of parametrial invasion are incipient infiltration of pericervical fascia (usually in depth ≤5 mm), grade 2; nodular infiltration of parametrium, grade 3, discontinual parametrial involvement (“skip-metastasis”), grade 4
[33]. Metastatic visceral paracervical lymph nodes are considered discontinual parametrial invasion on ultrasound (grade 4)
[34]. TNM and FIGO do not define how to classify metastases in the para-uterine visceral lymph nodes
[12][65]. Since the lymphatic spread from cervical cancer is initially to these visceral (parametrial) lymph nodes drained by internal iliac vessels (e.g., uterine vessels), their involvement could be classified as locoregional lymph node metastases and not as infiltrated parametria. Parametrial invasion towards the pelvic side wall upstages the disease from T2b to T3b
[12][65]. The pelvic side wall is defined as the parietal muscles of the lesser pelvis (obturator internus, coccygeus, and piriformis muscle), fascia, neurovascular structures, or skeletal portions of the bony pelvis. Pelvic side wall invasion by a tumour is a frequent cause of ureteric obstruction associated with hydronephrosis (
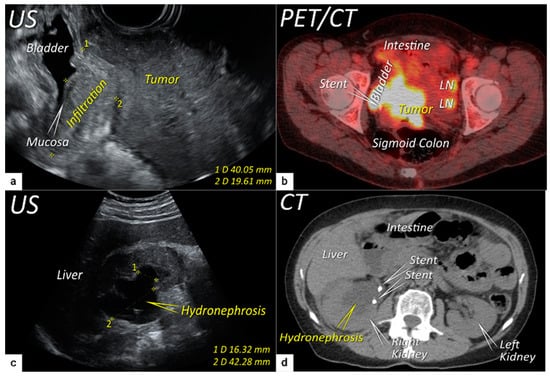
Figure 5). Additional use of transabdominal ultrasound is essential for screening of hydronephrosis with a sensitivity of 76.5%, specificity and positive predictive value of 100%, negative predictive value of 85%, and accuracy of 90%
[43]. The location of ureteric obstruction can be easily identified using a combination of transabdominal and transvaginal/transrectal ultrasound or MRI following the visualisation of suprastenotic dilatation of the ureter. Hydronephrosis on MRI can be examined if the protocol includes a sequence with an extended field of view to both kidneys in the axial or coronal plane. The degree of hydronephrosis is divided into three grades, as has been previously described
[33].
Figure 5. Infiltration of the right ventral parametria and hydronephrosis. The sagittal plane of the transrectal ultrasound (US) (a) demonstrating infiltration of the right ventral parametrium and the bladder wall (except for mucosa marked with an arrow) by a large squamous cell cervical carcinoma of 94 mm in the largest diameter in a 65-year-old woman with T3bN1M0/FIGO stage IIIC1. The transverse plane of fluorodeoxyglucose (FDG) -positron emission tomography-computed tomography (PET-CT) (b) in the same patient demonstrating infiltration of the bladder and sigmoid colon wall and metastatic lymph nodes in the left obturator fossa (LN). The sagittal plane of a transabdominal ultrasound scan in the same patient (c) demonstrating right kidney hydronephrosis (second grade) due to locally advanced cervical carcinoma with partial obstruction of the right ureter causing dilated renal calyx (1) and pelvis (2). The transverse plane of non-contrast CT (part of the FDG PET-CT examination) depicts second-grade hydronephrosis in the right kidney after placement of a ureteral stent in the same patient (d). CT, computed tomography; FDG PET-CT, fluorodeoxyglucose positron emission tomography fused with computed tomography; US, ultrasound.
2.4. Extension to Surrounding Organs (Bladder, Rectum, Sigmoid Colon)
The last step in local staging focuses on the assessment of tumour growth into the adjacent organs (bladder and rectum or sigmoid colon). The level of infiltration of both the bladder and rectum can be determined simultaneously using an identical grading system (see below)
[33]. Ultrasound or MRI are used to detect the infiltration of the endopelvic fascia based on the assessment of the contact planes between adjacent organs and the extent of involvement of both the bladder and rectal wall (
Figure 6 and
Figure 7)
[33][40].
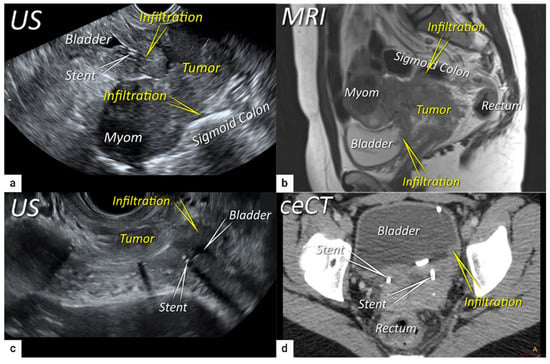
Figure 6. Infiltration of bladder and rectosigmoid colon wall. The sagittal plane of a transrectal ultrasound (US) (a) and T2-weighted magnetic resonance imaging (MRI) (b) depicting a squamous cell cervical carcinoma with necrotic components in a 38-year-old woman (T4N1M0/FIGO stage IVA) having tumour infiltrating the bladder and sigmoid colon wall including mucosa. Transverse plane of transrectal ultrasound (c) and axial contrast-enhanced computed tomography (d) showing deep bladder invasion in the same patient treated with bilateral ureteral stents. The deepest infiltration was seen on the left side of the bladder.
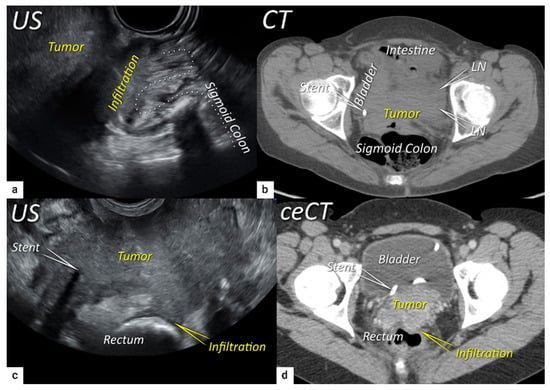
Figure 7. Infiltration of the rectosigmoid wall. Upper row: Sagittal transrectal ultrasound depicting a large squamous cell cervical carcinoma with a maximum diameter of 94 mm (T3bN1M0/FIGO stage IIIC1) in a 65-year-old woman (same patient as Figure 5), infiltrating the rectosigmoid colon with retraction of loops towards the tumour (a). Axial non-contrast computed tomography (CT) (b) shows a tumour infiltrating the muscle wall of the sigmoid colon and bladder and the pelvic side wall bilaterally with bulky lymph nodes in the left obturator fossa (LN). The right ureter with a stent is embedded within the right lateral pelvic side wall invasion. Lower row: A 38-year-old patient with maximum diameter 48 mm (T4N1M0/FIGO stage IVA) (same patient as in Figure 6) with squamous cell carcinoma depicted in the transverse plane by transrectal ultrasound (c) showing infiltration of the muscle layer of the rectum from the left uterosacral ligament and bilateral pelvic side wall infiltration with a visible right ureteral stent within the pelvic side wall infiltration. The transverse plane of contrast-enhanced computed tomography (CT) (d) demonstrates rectal wall infiltration and ureteral stent laterally. US, ultrasound; MRI, magnetic resonance imaging; ceCT, contrast-enhanced computed tomography.
In a single-unit study by Iwamoto et al., the accuracy of ultrasound in the detection of bladder infiltration was superior (95%) to other methods (76% for CT, 86% for cystoscopy, and 80% for MRI)
[39]. It has been proposed that the better results achieved with ultrasound are related to the dynamic aspect of the ultrasound examination; thanks to the positive sliding effect between organs, the authors could exclude bladder wall infiltration. The same manoeuvre can be used to exclude rectosigmoid wall infiltration. Huang et al. described ultrasound characteristics representative of the different stages of bladder wall invasion
[40]. In 2011, Fischerova categorised the infiltration of the bladder wall on ultrasound examination using four stages (0–3), from uninterrupted endopelvic fascia to complete disruption of the hyperechogenic mucosa with the presence of intraluminal tumour nodules
[33]. The same grading system can be used to assess the depth of rectal or sigmoid wall involvement
[33].
Ultrasound and MRI detect sequential changes in all the layers of the bladder or rectosigmoid wall with high accuracy, offering more information on the disease burden than the endoscopic examination (cystoscopy, rectoscopy), which only depicts the worst stage of infiltration (infiltration of the mucosa)
[40][66][67]. In a meta-analysis published in 2020 by Woo et al., the pooled sensitivity and specificity of five studies using MRI in bladder wall infiltration assessment were 84 and 95%, respectively
[54]. The minimally invasive diagnostic techniques, cystoscopy or rectoscopy, can be considered redundant because the results of these examinations do not change the management and only delay the treatment initiation. For that reason, the joint ESGO-ESTRO-ESP cervical cancer guidelines recommend cystoscopy and rectoscopy only for obtaining a biopsy in cases of suspected synchronous secondary malignancy
[9].
To sum up the local workup and present literature, the reported slightly better accuracy of ultrasound over MRI to delineate the tumour may be due to the combination of sonomorphology corresponding to features of different histological subtypes and evaluation of tumour vessels by Doppler defining the extent of the tumour growth within the cervix, as well as the use of suboptimal MRI protocols (without DWI) according to current imaging guidelines
[24]. The slightly better results for ultrasound in comparison to MRI (also without DWI) in parametrial invasion assessment may be firstly due to the recent technical improvements in ultrasound technology, including high-frequency endoluminal probes enabling detailed visualisation of the cervix contour and pericervical fascia. Secondly, it may be due to the Doppler scan’s discriminating accurately between cervical vessels and tumour spiculae into the parametria. Thirdly, it may be related to the possibility of performing dynamic evaluation during the ultrasound examination. Using a slight pressure of the probe against the cervix, the ultrasound expert can easily observe the sliding of the tumour against the surrounding tissue (positive sliding sign) and exclude their infiltration. Lastly, based on the excellent tissue plane resolution and dynamic aspect of examination, ultrasound excludes or confirms the infiltration of the bladder or rectum and precisely assesses the tumour depth of infiltration in the bladder/rectal wall. In accordance with the current evidence, the joint ESGO-ESTRO-ESP cervical cancer guidelines accepted transvaginal/transrectal ultrasound (TRS/TVS) as an effective alternative to the pelvic magnetic resonance (T2WI and DWI-MRI) in the primary workup of cervical cancer
[9][10]. Moreover, the revised FIGO staging emphasised that ultrasound in the hands of experienced operators can provide comparable information to MRI for the staging of cervical cancer
[12].