Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Agriculture, Dairy & Animal Science
Birds (Aves) are the most speciose of terrestrial vertebrates, displaying Class-specific characteristics yet incredible external phenotypic diversity. Critical to agriculture and as model organisms, birds have adapted to many habitats. The only extant examples of dinosaurs, birds emerged ~150 mya and >10% are currently threatened with extinction.
- avian genome
- avian karyotype
- interchromosomal rearrangements
- intrachromosomal rearrangements
1. Avian Biology and Its Importance
With around 10,500 extant representatives, birds are the most species-rich of tetrapod vertebrates [1]. Modern birds belong to the phylogenetic Class Aves and the subclass Neornithes. They are characterized by a combination of features not seen together in other vertebrates including homeothermy, flight (except for penguins, ratites and some others who have lost the ability), oviparity, nesting, the presence of a beak (without teeth), a high metabolic rate, feathers, and a lightweight skeleton. Occupying almost all terrestrial and many aquatic habitats, birds have adapted to a range of climate extremes from inland Antarctica to the tropics. The highest levels of species diversity is seen in tropical regions [2]. The phenotypic diversity seen in birds is extraordinary; sizes range from the bee hummingbird (Mellisuga helena) at approximately 5 cm in length to the ostrich (Struthio camelus), which stands at over 2 m tall. Birds have a high core body temperature (39–41 °C), high blood glucose levels, and energy expenditure levels that are five or more times higher than those commonly seen in mammals. Comparisons with similar sized mammals show that birds tend to live longer, despite the higher energy use [3]. Birds are social, with varying degrees of communication complexity including the use of calls and song (in some cases communicating by visual display); they can be socially cooperative, exhibiting behaviors such as flocking and mobbing. Most birds also provide an extended period of parental care that is often shared between parents and/or with other birds [4][5][6].
Birds are critical to agriculture (both meat and eggs) and are also model organisms for studies of virology, immunology, and developmental biology, e.g., [7]; they can also be valuable companion animals for humans. From an evolutionary point of view, the Aves Class is the only extant example of the Dinosauria (Theropoda) clade, e.g., [8], sharing a common ancestor with mammals around 310 million years ago (mya). In addition, approximately 1400 extant birds (>10%) are currently listed as threatened with extinction, with over 160 species becoming extinct in the last 500 years [9]. Many of these extinctions are considered to be a result of anthropogenic climate and habitat change, in particular due to the introduction of alien species such as rats into island habitats [9]. Further understanding of birds from an evolutionary point of view is therefore crucial to understand vertebrate evolution and to protect current species from further risk.
2. Avian Evolution
Originating around 150 mya in the late Jurassic, birds evolved from a theropod dinosaur lineage [10][11] at a time when the supercontinent Pangaea was separating into two landforms (Laurasia and Gondwana). The fossil Archaeopteryx lithographica dates back to 150 mya, was found in the 19th century in late Jurassic limestone in Germany [12], and provides evidence of a transitional species between ancient dinosaurs and modern birds. Although previously considered to be the fossil representative of an early modern bird, features such as a bony tail and teeth rule out A. lithographica from being considered a true avian ancestor [13].
The oldest unambiguous fossil representative of Neornithes (modern birds), Vegavis, is an aquatic bird classified within Anseriformes and most closely related to Anatidae—ducks, geese, and swans [14]. Dating back to ~67 mya, the discovery of this fossil supports the notion that representatives of modern birds were co-extant with non-avian dinosaurs prior to the Cretaceous–Paleogene (K-Pg) boundary 66 mya [14]. The inherent difficulties in fossil dating due to geographic and depositional sampling bias have led to much controversy in the field of paleontology [10], meaning that analyses at a genomic level are a useful complement to a fossil record that may imperfectly represent actual avian ancestors. As a result, the dinosaur ancestor of birds is generally considered to be bipedal, terrestrial, and relatively small (small size being a pre-adaptation to flight) with a limited flying ability, not dissimilar to the Galliformes [15].
The study of genomics has revolutionized avian phylogenetic studies and, until the publication of a revised avian phylogeny by Jarvis et al. [16], the timing of avian diversification was the subject of much debate. The first avian divergence is now considered to have taken place around 100 mya when the Palaeognathae (ratites and tinamous) diverged from the Neognathae (Galloanserae and Neoaves). Within the Palaeognathae, the ratites and tinamous then diverged 84 mya, while the Neognathae diverged into its stem lineages, the Galloanserae and Neoaves, 88 mya. The Galloanserae divergence into the Galliformes (landfowl) and Anseriformes (waterfowl) occurred around the time of the K-Pg extinction event 66 mya, with major divergences of the Neoaves into Columbea and Passerea now dated to before the K-Pg boundary (67–69 mya). The rest of the divergences within Neoaves were largely complete at the ordinal level by 50 mya, with the Passeriformes basal split estimated to have occurred approximately 39 mya [16]. The K-Pg event was a period of abrupt, mass global extinction and extreme climate change, coinciding with the Chicxclub asteroid impact in Mexico [17], extremely significant for archaic birds (Ornithurae), of which the Neornithes are descendants. Recent fossil evidence points to a major radiation of advanced ornithurines occurring prior to the end of the Cretaceous period. The same group then suffered an abrupt extinction around the K-Pg event, with their disappearance from the fossil record from the Paleogene period onwards [18]. Genomic data from Jarvis et al. [16] also suggest that the K-Pg transition period was one of rapid Neornithine speciation, with 36 lineages radiating over a period of 10–15 million years. Jarvis et al. propose that these revised dates challenge some of the previously held assumptions that Neornithine lineages diversified explosively significantly after the K-Pg boundary [16].
3. Defining the Avian Karyotype
The karyotype of any eukaryote essentially defines its overall genomic structure. It allows gross genomic differences to be compared between species, ultimately building an evolutionary tree of gross genomic changes. Defining the avian karyotype in molecular cytogenetic terms is however notoriously difficult, largely due to the presence of a (usually) large number of morphologically indistinguishable microchromosomes [19]. Classification of the larger macrochromosomes (up to chromosome 9, including the sex chromosomes) is possible using classical cytogenetic techniques such as standard karyotyping but, beyond this size, it is near impossible to complete for most species—hence the publication of partial, rather than full, avian karyotypes in all cases apart from chicken [20]. Even at the macrochromosomal level, chromosome banding can be difficult to identify, thereby making a robust analysis of cross-species homology difficult and unreliable. The development of chromosome paints derived from the amplification and fluorescence labelling of chicken macrochromosomes has improved on this limited resolution and led to the publication of cross-species analysis (“zoo-FISH”) data including approximately 120 avian species from 22 different orders [19]. These studies are, however, restricted to analysis of the macrochromosomes. Some success has been achieved using microchromosomal chromosome paints, e.g., [21]; but, again, this is limited, largely due to the inability to separate each microchromosome by flow cytometry—the starting point for the generation of chromosome paints. A degree of success using a cross-species BAC mapping approach was originally reported, although this was limited to closely related species, with 70% success rates reported using chicken BACs on turkey (Mealeagris gallopavo) [22] reducing to under 40% when tested on duck (Anas platyrynchos) [23]. Marginally higher rates between chicken and duck have been reported elsewhere, however [24]. Up until the earliest years of the millennium, the apparent highly conserved nature of microchromosomes proved difficult to investigate using either classical or molecular cytogenetic methods, e.g., [22][23].
4. Avian Karyotypic Diversity
The highly distinctive, ‘signature’ avian karyotype is typically divided into around 10 macrochromosome pairs and around 30 pairs of evenly sized, morphologically indistinguishable microchromosomes [20][25][26][27][28][29][30][31]. The morphological similarity of the microchromosomes, and the sheer number of them, makes a full classical karyotype almost impossible to generate and analyze. In fact, although over 1000 karyotypes [19] have been published to date for a class that represents around 10,500 extant species, these are partial at best, with only 5–10 pairs of chromosomes easily identifiable. Rare exceptions to this ‘avian style’ signature karyotype include those with an unusually small diploid number such as the stone curlew (Charadriiformes, Burhinus oedicnemus; 2n = 42) [32] and the beach thick knee (Charadriiformes, Esacus magnirostris; 2n = 40) [25]; and those with an uncommonly high diploid number such as the kingfishers (Coraciiformes, Alcedo atthis; 2n = 132) [33] and hoopoes (Bucerotiformes, Upupa epops; 2n > 120) [25]. It is essential to note however that these deviations are not necessarily uniformly representative of their entire avian orders (e.g., Charadriiformes, Coraciiformes, and Bucerotiformes) (reviewed in [21]). Therefore, exceptions are not the rule within these orders. For instance, Ciconiiformes (storks), Pelecaniformes (ibis, herons, pelicans, hamerkop, and shoebill), Falconiformes (falcons), and Psittaciformes (parrots) usually exhibit lower diploid numbers (reviewed in [19]) but patterns differ, while toucans (e.g., Ramphastos toco; 2n = 114) defy the norm with usually higher diploid numbers [34].
At a molecular level, microchromosomes are particularly unique in being extraordinarily GC-rich and gene-dense, whilst accounting for only 23% of the genome but 48% of the genes [35][36][37][38]. Notably, in birds and snakes, microchromosomes also display a low transposable element content and high rates of recombination [39][40][41]. Burt [38] proposed that the microchromosomes present in birds were established in the ancestral vertebrate karyotype 400 mya. This appears to be supported by Nakatani and colleagues’ [42] study, which found that many avian microchromosomes corresponded directly with gnathostome ancestor protochromosomes. In turn, this implied that the characteristic avian karyotype was established at an extraordinarily early stage of evolution (see Section 9).
5. Sex Chromosomes in Birds
Unlike mammals, birds exhibit the highly conserved ZW sex chromosome system, with females being heterogametic (ZW) and males homogametic (ZZ) [43][44]. In all Neognathae, the Z and W chromosomes are differentiated in terms of size and morphology, with the W being largely heterochromatic, gene poor, and significantly smaller than the Z [45][46][47]. Exceptions to this rule include a few cases reviewed in Schartl et al. [48], where the W chromosome is heterochromatic and the same size as, or even bigger than, the Z chromosome. Ratites, however, have a W chromosome of a similar size to the Z and it is homologous in its entirety with the exception (in the case of emus) of a small region near the centromere [49][50][51]. It has been suggested that the alteration of chromatin conformation induced by transposable element (TEs) accumulation comprises an important early step in sex chromosome differentiation [52]. Despite the difference in size, it can be inferred that the ZW system was present prior to the divergence of the Palaeognathae and Neognathae lineages [53], but that the differentiation in size between the two chromosomes occurred afterwards. Although superficially resembling the XY system seen in mammals, the XX/XY (mammalian) and ZZ/ZW (avian) systems exhibit no homology [54] and have completely independent origins. The avian Z chromosome shares homology with human autosomes 5, 9, and 18 [55]. The human/mammalian X chromosome, on the other hand, shares homology with a block of the q-arm of chicken chromosome 1 and a 20 Mb portion of the p-arm of chicken chromosome 4 (a microchromosome in most other birds) [56]. The sex-determining gene in birds is not SRY as in mammals (the homologue of which in fact lies on chicken chromosome 4) [57]. Instead, it has been suggested that the gene DMRT1 found on the Z chromosome may be the key to sex determination using a dosage-dependent system. Male determination requires two copies of the gene as found in ZZ males, and DMRT1 has also been shown to be required for testis formation [58]. There is still much debate, however, as to what determines sex in birds, with possible candidates (among other theories) including W-specific genes that may determine ovarian function [57]. Improvements in the assembly of the Z chromosome have been achieved using a BAC-based approach [55], along with further work to improve the assembly of the W chromosome [59].
Recent studies revealed a surprising dynamism in avian sex chromosomes, challenging the earlier perception of their stability. One remarkable discovery involved the identification of a multiple-sex chromosome system (♂Z1Z1Z2Z2/♀Z1Z2W) in the penguin species Pygoscelis adeliae (Sphenisciformes) [60]. Additionally, instances of independent autosome–sex chromosome fusions have been identified in Sylvioidea species through the analysis of genomic data [61]. Moreover, neo-sex chromosomes have been identified in parrots [62], while similar findings were observed in a cuckoo species [63]. These findings collectively highlight a previously unrecognized diversity and dynamism within avian sex chromosome systems.
6. Chromosomal Rearrangements in Birds
As described above, a key feature unique to birds is the high level of karyotypic stability. That is, the majority (~70%) of avian species have a karyotype that is very similar in terms of size and gross genomic structure to that of the chicken (2n = 78). Exceptions to this rule include the Ciconiiformes (storks), Pelecaniformes (ibis, herons, pelicans, hamerkop, and shoebill), Falconiformes (falcons), and Psittaciformes (parrots), which have lower diploid numbers than chickens, fewer microchromosomes, and thus evidence of chromosomal fusion (reviewed in [19]). The use of chromosome paints derived from chicken flow-sorted chromosomes demonstrated a high degree of conservation between the macrochromosomes. This supports the view that the avian genome structure is highly conserved, even across large phylogenetic distances. Technical difficulties creating microchromosomal paints have limited the scope of their use for this analysis. Recently, a series of papers using a microchromosomal BAC-based FISH approach found evidence of microchromosomes fusing to macrochromosomes in a few avian orders, thereby filling in gaps that had been unassigned, e.g., [21][64].
In contrast to mammals, birds therefore exhibit a slow rate of change in interchromosomal rearrangements [65][66][67]. Despite this apparently slow rate, it is likely that the same does not apply to intrachromosomal rearrangements, which are seen considerably more frequently [68][69][70]. A comparison of the genomes of the chicken, turkey, and zebra finch and analysis using the Genalyzer tool [71] revealed a high degree of intrachromosomal rearrangement within the macrochromosomes, many of which were subsequently confirmed by FISH. Analysis of intrachromosomal rearrangements in the microchromosomes, however, has been limited to a few studies and few avian orders. The first investigation by Rao et al. [72] used the radiation hybrid method to assemble the duck genome and also compared the microchromosomes of the duck to those of the chicken. A second study by Lithgow et al. [21] found no interchromosomal rearrangements between chicken, turkey, and zebra finch microchromosomes, but found multiple intrachromosomal changes.
In 2004, the chicken became the first avian species for which a whole genome sequence was generated [73] and a karyotype fully defined [20]. At that time, chromosome paints were generated from microdissected metaphase preparations to identify all chromosomes uniquely. Subsequent efforts to sequence DNA from these clones, however, proved unsuccessful [74]. Moreover, the original chromosome paint probes from Masabanda et al. [20] have since degraded [74]. Reliable tools for the detection of these chromosomes were developed that contributed to identifying chicken microchromosome syntenies across many avian groups [75]. Indeed, until recently, the very smallest of the microchromosomes, the ‘D group’ (chromosomes 33–39) [20], had no sequences associated with them in the genome assembly (Figure 1a). At the time of writing, all chicken macro- and microchromosomes now have respective sequences assigned to them and are annotated thanks to the recent work of Huang et al. [76], although there are still 172 scaffolds to be placed or localized (Figure 1b). The chicken chromosome-level assembly is the most widely used reference genome in the avian comparative genomic field for the cytogenomic and phylogenomic analyses of birds.
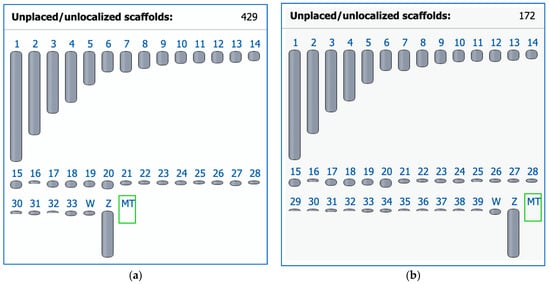
Figure 1. Ideogram view in the NCBI Genome Data Viewer for two chicken (Gallus gallus, GGA) representative genome assemblies. (a) The previous assembly GRCg6a (GCF_000002315.6) released on 27 March 2018 with 1 to 33 autosomes. (b) The latest assembly bGalGal1.mat.broiler.GRCg7b (GCF_016699485.2) as of 19 January 2021 has 1 to 39 autosomes (including macrochromosomes GGA1–GGA9 and microchromosomes GGA10–GGA39) and fewer unplaced scaffolds as a result of work later published in Huang et al. [76]; Z and W, sex chromosomes; MT, mitochondrial genome.
Unlike research performed on mammalian chromosomes, hybridization across a greater evolutionary distance (i.e., beyond the phylogenetic Class) is possible with chicken chromosome paints. For example, homology has been detected between chicken, turtles, and crocodiles, all of which last shared a common ancestor over 250 mya [77][78]. The use of microchromosomal paints, however, has been comparatively limited [32][49][79], largely due to the paints being divided into ‘pools’ of microchromosomes rather than being assigned to separate, entire chromosomes [21]. Whilst able to define whole blocks of homology between species, the orientation of the blocks cannot be defined using chromosomes nor can intrachromosomal rearrangements between species. To overcome both limitations, a BAC-based approach was necessary, either in conjunction with chromosome paints or in isolation.
7. Closer Examination of Avian Microchromosomes
As described above, most of the avian comparative genomic studies performed to date at a chromosomal level have been limited to investigating macrochromosomal chromosome painting because microchromosomal paints were not available. The development of BAC libraries as a product of genome sequencing, e.g., [75][76][80] has, however, facilitated the development of a set of BACs that have been selected that successfully hybridize to chicken microchromosomes. Using BACs derived from the (sequenced) microchromosomes in combination with the macrochromosomal chicken paints therefore allows molecular cytogenetic examination of almost the entire chicken genome and its chromosomal homologs in other species.
Identification of the genomic features unique to these BACs using a bioinformatic approach [75][81] led to a refinement in the methods used to select BACs designed to hybridize across multiple species [8][70][75][82][83][84]. This resulted in an improvement in hybridization rates between species by several orders of magnitude. Selection of the BACs was based on successful hybridization across five core avian species [70][75] and by the position of each BAC in the reference species (at the most distal region of each chromosome). These BACs provided a consistent anchor point from which to compare species to track chromosomal rearrangements over time. As reported by Damas et al. [75], rather than being limited to comparing multi-species’ chromosomal rearrangements within a specific order, this approach allowed for comparison across an entire Class (and, to some degree, even beyond this [75][81]).
As the best-characterized avian species with the largest number of BAC libraries available, e.g., [83], the chicken was used as the reference avian species in most of the studies. BACs located in the most distal (where possible) regions of the p- and q-arms of chromosomes 1–28 were selected according to the selection criteria described by Damas et al. [75]. The co-hybridization of p- and q-arm BACs for each chromosome was performed to verify correct mapping of the BACs, producing bright punctate signals for each of the chicken chromosomes, an example of which is shown for chicken chromosome 12 (GGA12) in Figure 2.
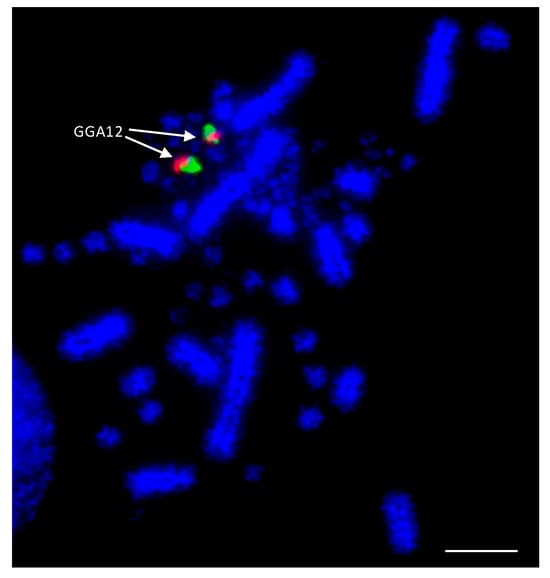
Figure 2. Example of dual FISH results for chicken (Gallus gallus—GGA) chromosome 12 to confirm correct mapping. The p-arm BAC (CH261-88K1) is labelled with FITC (fluorescein isothiocyanate) (green) and the q-arm BAC (CH261-152H14) is labelled with Texas Red (red). Scale bar 10 μm.
Clear, punctate signals (similar to those seen on the chicken metaphases) were achieved for all microchromosome BACs for each species (apart from the two BACs for chicken chromosome 25 when tested on Passeriformes representatives that did not produce a signal). Clear signals were achieved for all macrochromosome BACs with a few individual species-specific exceptions.
Using this set of cross-species probes, specifically to investigate the microchromosomes, permitted analysis at a higher resolution than previously achieved. In most studies, two BACs were selected from each of the sequenced chicken microchromosomes (from GGA10 to GGA28, except for GGA16) and dual FISH was performed on a total of 42 avian species. In all species tested, regions homologous to chicken chromosomes 22, 24, 26, and 27 appear to have remained intact as entire microchromosomes with no evidence of chromosomal fusion [64][84]. Figure 3 shows representative images for chicken chromosome 24 tested on multiple species with the BACs illustrating that this chromosome appears to remain intact as a microchromosome in all species tested with no sign of interchromosomal rearrangement.
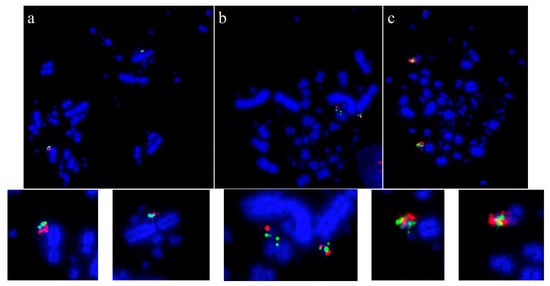
Figure 3. Microchromosomal conservation observed across a wide range of avian species as revealed by testing BACs from chicken chromosome 24 (CH261-103F4 FITC in green and CH261-65O4 Texas Red in red): Phalacrocorax brasilianus (a), Crotophaga ani (b), and Geotrygon montana (c). Frame enlargements immediately beneath, occasional multiple signals (e.g., in (b,c)) as commonplace in FISH experiments and representing small amounts of background hybridization.
7.1. Species with No Apparent Interchromosomal Rearrangement between the Microchromosomes
No apparent changes from the ancestral microchromosomes appear to have occurred among the representatives from the following orders: Galliformes, Gruiformes, Anseriformes, Columbiformes, Otidiformes, Piciformes, Trogoniformes, Strigiformes, and Struthioniformes (Figure 4). The microchromosomes of each bird remain conserved in the same pattern exhibited in the chicken with BACs hybridizing consistently together across all species tested despite there being over 100 million years since they diverged.
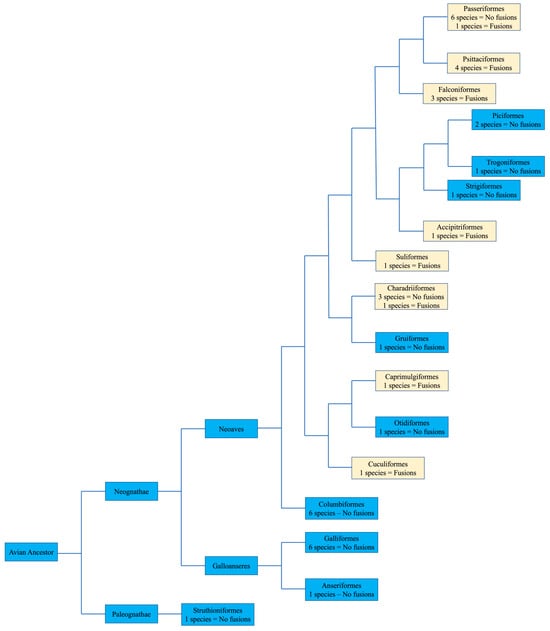
Figure 4. Bird phylogeny illustrating the presence or lack of interchromosomal rearrangement involving microchromosomes based on BAC FISH. The numbers of species with or without interchromosomal rearrangement involving microchromosomes is illustrated in each order. Macrochromosomal fusions are not listed. Phylogenetic relationships followed Jarvis et al. [16]. Light colored boxes indicate where interchromosomal changes occurred.
7.2. Species Demonstrating Rearrangement between Microchromosomes
7.2.1. Psittaciformes
Among the Psittaciformes, four species have been investigated using microchromosome BACs probes: the kakariki (Cyanoramphus novaezelandia), the cockatiel (Nymphus hollandicus), the budgerigar (Melopsittacus undulatus), and the monk parakeet (Myiopsitta monachus). Shared rearrangements were not observed between these species [64][81][85]. Fusion events were detected for the homologs for GGA10, 11, and 14 in the kakariki, the cockatiel, and the budgerigar with the additional fusion observed of the GGA13 homolog in the budgerigar. The monk parakeet showed several tandem fusions between microchromosomes and fusions between macrochromosomes and microchromosomes, resulting in a karyotype with the low diploid number of 48 [85]. An example of the microchromosome fusion in the monk parakeet genome is shown in Figure 5.
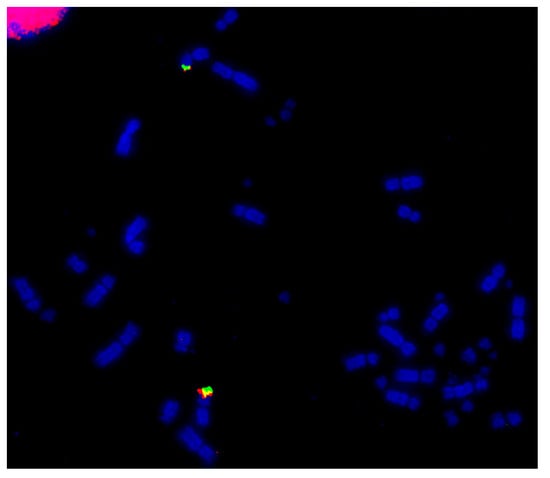
Figure 5. Hybridization of GGA22 BACs (CH261-40J9-FITC in green and CH261-18G17-Texas Red in red) to monk parakeet (Myiopsitta monachus, MMO) metaphase illustrating fusion of the ancestral microchromosome to a macrochromosome.
Figure 6 shows the overall karyotypic structure of the cockatiel and illustrates that, despite broadly similar patterns of rearrangement, there are fewer rearrangements that have occurred between the macrochromosomes when compared to the budgerigar. The kakariki karyotype appears most similar to the budgerigar but requires further mapping to confirm the overall structure as there are no previously published studies for this species. Macrochromosomal rearrangements are based on those previously established through chromosome painting studies by Nanda et al. [86] and confirmed by BAC FISH.
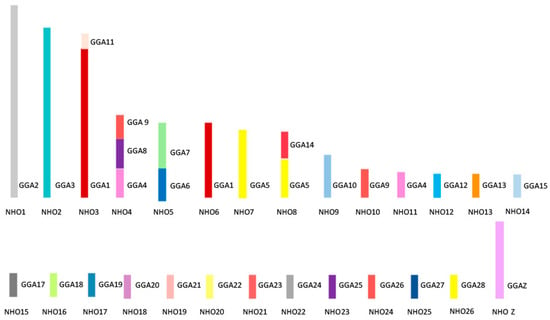
Figure 6. Ideogram representing karyotypic structure of the cockatiel (Nymphus hollandicus, NHO) illustrating an overall structure. Each chicken (GGA) homolog is represented as a different color.
7.2.2. Falconiformes
Among the Falconiformes, extensive rearrangement appears to have taken place with regions homologous to GGA microchromosomes 10, 12, 13, 14, 15, 17, 18, 19, 20, 21, 23, and 28 fused to GGA macrochromosome regions [81][82]. An example of this is illustrated in Figure 7 where GGA18 homologs are fused to a macrochromosome in the peregrine falcon. Lineage-specific rearrangements were apparent with no evidence of chicken chromosome homologs 15, 18, 19, 23, and 28 being rearranged in any of the other (non-falcon-related) species tested. Interestingly, 15, 18, and 19 appear to have fused together as one chromosome (to chicken homolog 4) in both falcon species tested, while 23 and 28 have both fused to the homolog of chicken chromosome 2, which, at some point (either pre- or post-fusion) has split into two chromosomes. Both of the falcon species tested (peregrine and saker) appear to exhibit the same pattern of rearrangement (with the exception of peregrine chromosome 1 for which there is a centric fusion), suggesting that any lineage-specific rearrangements rapidly became fixed in the population with little interchromosomal rearrangement since. In addition, there appears to be no interchromosomal rearrangement between each pair of BACs tested, suggesting that these regions of DNA are highly conserved and not prone to breakage.
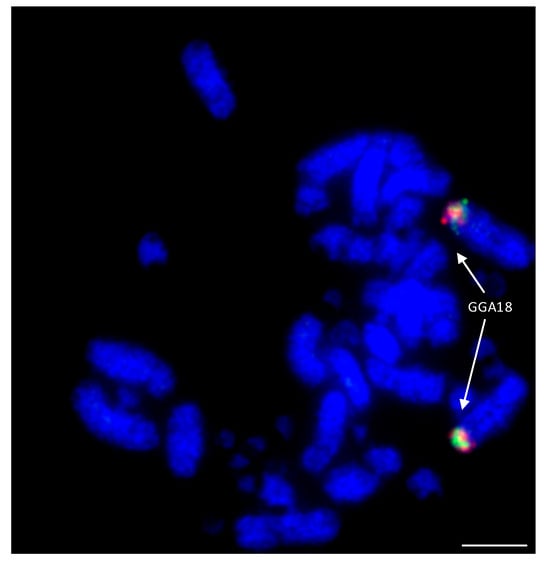
Figure 7. Hybridization of GGA18 BACs (CH261-60N6-FITC in green and CH261-72B18-Texas Red in red) to peregrine falcon (Falco peregrinus) metaphases illustrating fusion of ancestral microchromosome to a macrochromosome. Scale bar 10 μm.
Figure 8 illustrates the overall karyotype structure of the peregrine falcon tested showing extensive interchromosomal rearrangement between the micro- and the macrochromosomes [81][82]. The karyotype of the saker falcon follows the same pattern with the exception of peregrine chromosome 1 [81]. In the saker falcon, this chromosome has split into two chromosomes with the breakpoint occurring within the region of the GGA5 homolog. This suggest that this is a fission that has occurred after the falcon karyotype was formed rather than a later peregrine-specific fusion.
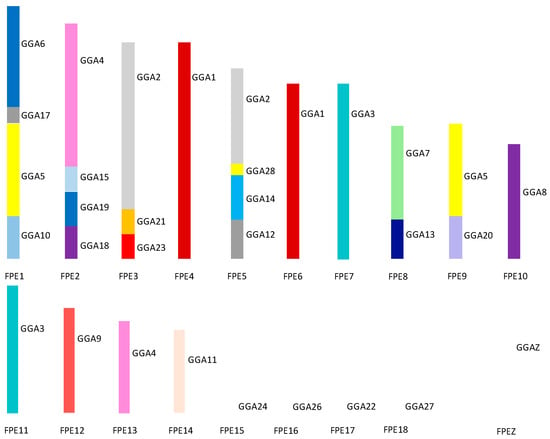
Figure 8. Ideogram representing overall karyotypic structure of the peregrine falcon (Falco peregrinus—FPE) illustrating an extensive amount of interchromosomal rearrangement throughout the karyotype. Each chicken (GGA) homolog is represented as a different color.
7.2.3. Ciconiiformes
Most of the reports about the chromosome organization of storks (Ciconiiformes) have relied solely on analyses by conventional staining; however, an interesting variation in the diploid chromosome number was found (2n = 52 to 78) [reviewed in 21]. Considering that most species have similar macrochromosomes, some authors propose that karyotype evolution mainly involves fusions between microchromosomes, which were later confirmed by chromosome painting [87]. Nevertheless, the exact microchromosomes involved in the rearrangements were not identified and this question therefore warrants additional studies.
7.2.4. Pelecaniformes
Molecular cytogenetics is still scarce in this order; however, recent studies using chromosome painting with chicken and/or stone curlew (B. oedicnemus, BOE) paints indicated that the karyotype of Pelecaniformes is reorganized, similar to that of the parrots and falcons [88][89]. The stone curlew chromosome painting in three Pelecaniformes species (Ardea cinerea, Egretta garzetta, and Nipponia nippon) indicated that different chromosome rearrangements occur in different Pelecaniformes lineages [88]. The main rearrangements were fusion events, including macro- and microchromosome. In Syrigma sibilatrix, the GGA8, GGA9, and GGA10 chromosome paints hybridized to the long arms of biarmed macrochromosomes, also indicating fusions with microchromosomes [89]. Although BOE and chicken (GGA) microchromosome paints do not allow for the identification of the exact microchromosome involved in the fusion events, these studies indicated that fusion events involving these tiny chromosomes are nonetheless frequent among Pelecaniformes. Future studies using BACs probes are therefore necessary.
7.2.5. Caprimulgiformes, Cuculiformes, Suliformes, and Passeriformes
While several studies demonstrated that interchromosomal rearrangements involving macro- and microchromosomes have played a role in the karyotype evolution of Ciconiiformes, Falconiformes, and Psittaciformes, recent studies have also observed this type of rearrangement in Caprimulgiformes, Cuculiformes, Suliformes, and Passeriformes species [84][90]. However, in these studies, only one representative species was investigated (Caprimulgiformes, Cuculiformes, and Suliformes) or the rearrangements were found only in one species (Passeriformes). Future studies are therefore necessary to explore whether these fusions are species-specific or are a common feature of each order. Most species of Passeriformes demonstrate the typical avian karyotype, as evidenced using microchromosomal BAC FISH, where only one species (Tolmomyias sulphurescens, 2n = 60, Rhynchocyclidae) from seven demonstrated microchromosomal fusions [90]. It is likely that the low diploid number is specific to the Rhynchocyclidae family, while remaining conserved in other Passeriformes species [90].
Among the Cuculiformes, Crotophaga ani is the only species to date to be investigated using BAC FISH, with extensive chromosome reorganization involving macro- and microchromosomes observed. A fusion between chicken chromosome 17 and Z was also found. Z–autosome Robertsonian translocations are rare in birds and have only been otherwise observed in Sylvioidea species (Passeriformes) [61][91][92][93] and in selected parrot species with a genome sequence [62].
8. Microchromosomes and their Conservation, Whether Discrete or Fused
Microchromosomal rearrangement has long been considered to occur rarely compared to other chromosomal rearrangements in birds. These highly gene-dense chromosomes are thought to have changed very little throughout the last 100 million years of avian evolution [94] with a high degree of conservation potentially dating back even further to the ancestral vertebrate 400 mya [38][42]. Prior to the publication of cytogenetic studies in the last few decades however, there has been little cytogenetic evidence to support the notion of this degree of conservation. What evidence is available was originally focused largely on closely related and karyologically similar species such as chicken and duck [23][24]. The BAC-based approach enabled analysis across avian representatives from 17 different orders, all of which share a common ancestor over 100 mya. These results clearly illustrate an extraordinary level of microchromosome conservation, with 9 out of the 17 orders exhibiting no apparent change from the microchromosomal pattern exhibited in the chicken. From a microchromosomal point of view, these results support the hypothesis proposed in a previous study [95] that the avian ancestor most closely resembled the chicken. Of the avian species that did exhibit microchromosomal rearrangements, the three representatives of the Falconiformes (the saker, peregrine falcon, and gyrfalcon) share the same pattern of fusion, from which it can be inferred that early in the evolution of this order there was a large degree of rearrangement that became fixed in the population [81][82]. It may therefore be that there is some biological advantage to this karyotypic structure for these birds, perhaps due to the high metabolic demands required by birds of prey. Of the other highly rearranged order, the Psittaciformes, the microchromosomal fusions exhibited in each of the species are not consistent with one another. This suggests that karyotypic evolution has continued from their common ancestor and that species-specific rearrangements are apparent [64][81].
In all these cases, however, it appears that there is a bias towards the microchromosomes remaining as discrete units, even when fused into highly complex karyotypes such as those of the Falconiformes and the Psittaciformes. As mentioned above, this same pattern is evident in the chicken, where the p-arm of chromosome 4 is a microchromosome in most other species, and thus ancestral. In the chicken, despite fusing to a macrochromosome, it remains intact, even retaining all its uniquely microchromosomal sequence characteristics such as a high GC and gene content [73].
Even considering these lineage specific rearrangements, there appear to be four microchromosomes (GGA22, 24, 26, and 27) that across all birds tested thus far remain conserved in their entirety, with no signs of apparent fusion. In the chicken, these are four of the smallest sequenced chromosomes with sizes ranging from 4 to 6 Mb. Further sequence analysis may reveal signature features of these chromosomes that may indicate a biological reason as to why these chromosomes are left intact. If there is any correlation with the size of the chromosomes and their lack of rearrangement, then this would suggest that the very smallest ‘D-group’ chicken microchromosomes (33–39) [20] are also less prone to chromosomal fusion. In fact, upon the exclusion of the two most rearranged lineages (parrots and falcons), a discernible pattern emerges where species from various orders appear to have accumulated primarily species-specific microchromosomal rearrangements rather than a shared characteristic. All other species analyzed exhibit the same pattern of conserved microchromosomal arrangement. Given that orders such as the parrots and the falcons are karyotypically the exception rather than the rule, this illustrates quite how profound this level of genome conservation really is.
In addition to the aforementioned attributes of microchromosomes, it is essential to highlight further distinctive features. Microchromosomes exhibit notable arrangements in the interior of the interphase nucleus, with macrochromosomes at the periphery [37][96][97][98]. Interestingly, studies of both avian and primate demonstrate that fusions involving gene-dense chromosomes with gene-poor ones do not appear to alter their nuclear positions [64][99][100]. A noteworthy characteristic is that microchromosomes also exhibit consistently high degrees of interchromosomal interaction (particularly with other microchromosomes), being co-localized in this central nuclear domain. This is observed across all microchromosomes in reptiles and birds [97][98], suggesting that this feature can be regarded as an ancestral trait. Interestingly, this persists even after their integration into a macrochromosome, albeit eroding over time [98]. Nevertheless, it is crucial to note that newly emerged microchromosomes swiftly establish high interactions with other microchromosomes [98], perhaps because they consist of a higher proportion of open chromatin compared to macrochromosomes. For a more comprehensive exploration of microchromosome properties, refer to the in-depth review by Srikulnath et al. [101].
9. Dinosaurian Origins of the Signature Avian Karyotype
Although the signature avian karyotype contains both macro- and microchromosomes, it would be wrong to suggest that the presence of both macro- and microchromosomes alone are a unique feature of avian genome organization. Indeed, microchromosomes are typical of most amniotes (many reptiles such as snakes, turtles, and lizards) with mammals and crocodilia (the only extant examples of non-avian archosaurs) being exceptions [102][103][104][105]. The greatest number and smallest size of microchromosomes are, however, typically found among birds. Burt [38] hypothesized that some microchromosomes were present in the common dinosaur ancestor that gave rise to birds (that probably had 2n = ~60) and that a series of fissions in the avian lineage resulted in the basic pattern of 2n = 80 (~30 pairs of microchromosomes) becoming fixed before the Palaeognathae–Neognathae divergence 100 mya.
Evidence provided by a number of studies, e.g., [81][95][106] leads to suggested possible mechanisms why, with relatively rare exceptions, avian genomes remain evolutionarily stable interchromosomally and have possibly done so through dinosaur and pterosaur lineages. The absence of interchromosomal rearrangement either suggests an evolutionary advantage to retaining this signature avian/dinosaur/pterosaur configuration, or else little opportunity for change. Evidence of considerable intrachromosomal change in pigeons [69][70][75] and Passeriformes species [68][70][95] provides evidence that intrachromosomal change proceeds largely unhindered and can accelerate in line with rapid speciation events. Indeed, the near absence of interchromosomal rearrangement is no barrier to diversity and a direct correlation has been reported between the rates of speciation and intrachromosomal rearrangement [107]. There may even be an evolutionary advantage to maintaining a karyotypic structure formed of many compact, gene-rich microchromosomes [95].
It is a reasonable assumption that the characteristically stable avian gross karyotypic structure has a reduced opportunity for chromosome rearrangement, as there are low numbers of recombination hotspots, fewer repeat structures such as transposable elements, and fewer endogenous retroviruses. All of these genomic features have been previously demonstrated to provide substrates for interchromosomal rearrangement and all are sparser in avian, compared to other genomes [94]. In previous studies it has been argued that the signature avian karyotype evolved in response to the shrinking of the genome in birds as a result of the metabolic demands of flight [108][109]. The results reviewed here, however, indicate that the basic karyotype structure was in place long before avian genome size reduction. The average genome size in non-dinosaur and non-avian saurians (lepidosaurs, turtles, and crocodiles) is around 3 Gb [110] and is significantly smaller in saurischian (1.78 pg) in comparison to ornithischian dinosaurs (2.49 pg) [111]. Although flight evolution may be a factor in genome size reduction, therefore (pterosaurs are reported to have smaller genomes than other Avemetatarsalians [112] and bats have smaller genomes than other mammals [113]), other factors are clearly in play as flight only evolved in therapods approximately 150 mya [114]. It is possible therefore that the evolution of the karyotype was a driver of genome size reduction rather than the other way around.
This entry is adapted from the peer-reviewed paper 10.3390/cells13040310
References
- Gill, F.; Donsker, D.; Rasmussen, P. (Eds.) IOC World Bird List (v12.2). 2022. Available online: https://www.worldbirdnames.org/new/ (accessed on 1 November 2023).
- Weir, J.T.; Schluter, D. The latitudinal gradient in recent speciation and extinction rates of birds and mammals. Science 2007, 315, 1574–1576.
- Holmes, D.J.; Ottinger, M.A. Birds as long-lived animal models for the study of aging. Exp. Gerontol. 2003, 38, 1365–1375.
- Farrar, V.S.; Flores, L.; Viernes, R.C.; Pereira, L.O.; Mushtari, S.; Calisi, R.M. Prolactin promotes parental responses and alters reproductive axis gene expression, but not courtship behaviors, in both sexes of a biparental bird. Horm. Behav. 2022, 144, 105217.
- Mérő, T.O.; Žuljević, A.; Lengyel, S. The role of reed management and habitat quality on brood parasitism and chick survival of the brood parasitic common cuckoo. Ecol. Evol. 2023, 13, e9705.
- Vanadzina, K.; Street, S.E.; Healy, S.D.; Laland, K.N.; Sheard, C. Global drivers of variation in cup nest size in passerine birds. J. Anim. Ecol. 2023, 92, 338–351.
- Beacon, T.H.; Davie, J.R. The chicken model organism for epigenomic research. Genome 2021, 64, 476–489.
- O’Connor, R.E.; Romanov, M.N.; Kiazim, L.G.; Barrett, P.M.; Farré, M.; Damas, J.; Ferguson-Smith, M.; Valenzuela, N.; Larkin, D.M.; Griffin, D.K. Reconstruction of the diapsid ancestral genome permits chromosome evolution tracing in avian and non-avian dinosaurs. Nat. Commun. 2018, 9, 1883.
- BirdLife International. We Have Lost Over 150 Bird Species Since 1500. 2017. Available online: http://www.datazone.birdlife.org/sowb/casestudy/we-have-lost-over-150-bird-species-since-1500 (accessed on 1 November 2023).
- Chiappe, L.M.; Dyke, G.J. The early evolutionary history of birds. J. Paleontol. Soc. Korea 2006, 22, 133–151.
- Dyke, G.; Kaiser, G.W. Living Dinosaurs the Evolutionary History of Modern Birds; Wiley Blackwell: Hoboken, NJ, USA, 2011.
- von Meyer, H. Archaeopteryx lithographica (Vogel-Feder) und Pterodactylus von Solnhofen. (Letter to Prof. Bronn of 30 September 1861). Neues Jahrb. Für Mineral. Geogn. Geol. Und Petrefaktenkunde 1861, 1861, 678–679.
- Mayr, G.; Pohl, B.; Hartman, S.; Peters, D.S. The tenth skeletal specimen of Archaeopteryx. Zool. J. Linn. Soc. 2007, 149, 97–116.
- Clarke, J.A.; Tambussi, C.P.; Noriega, J.I.; Erickson, G.M.; Ketcham, R.A. Definitive fossil evidence for the extant avian radiation in the Cretaceous. Nature 2005, 433, 305–308.
- Witmer, L.M. The debate on avian ancestry: Phylogeny, function, and fossils. In Mesozoic Birds: Above the Heads of Dinosaurs; Chiappe, L.M., Witmer, L.M., Eds.; University of California Press: Berkeley, CA, USA, 2002; pp. 3–30.
- Jarvis, E.D.; Mirarab, S.; Aberer, A.J.; Li, B.; Houde, P.; Li, C.; Ho, S.Y.; Faircloth, B.C.; Nabholz, B.; Howard, J.T.; et al. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 2014, 346, 1320–1331.
- Schulte, P.; Alegret, L.; Arenillas, I.; Arz, J.A.; Barton, P.J.; Bown, P.R.; Bralower, T.J.; Christeson, G.L.; Claeys, P.; Cockell, C.S.; et al. The Chicxulub asteroid impact and mass extinction at the Cretaceous-Paleogene boundary. Science 2010, 327, 1214–1218.
- Longrich, N.R.; Tokaryk, T.; Field, D.J. Mass extinction of birds at the Cretaceous-Paleogene (K-Pg) boundary. Proc. Natl. Acad. Sci. USA 2011, 108, 15253–15257.
- Degrandi, T.M.; Barcellos, S.A.; Costa, A.L.; Garnero, A.D.V.; Hass, I.; Gunski, R.J. Introducing the bird chromosome database: An overview of cytogenetic studies in birds. Cytogenet. Genome Res. 2020, 160, 199–205.
- Masabanda, J.S.; Burt, D.W.; O’Brien, P.C.; Vignal, A.; Fillon, V.; Walsh, P.S.; Cox, H.; Tempest, H.G.; Smith, J.; Habermann, F.; et al. Molecular cytogenetic definition of the chicken genome: The first complete avian karyotype. Genetics 2004, 166, 1367–1373.
- Lithgow, P.E.; O’Connor, R.; Smith, D.; Fonseka, G.; Al Mutery, A.; Rathje, C.; Frodsham, R.; O’Brien, P.; Kasai, F.; Ferguson-Smith, M.A.; et al. Novel tools for characterising inter and intra chromosomal rearrangements in avian microchromosomes. Chromosome Res. 2014, 22, 85–97.
- Griffin, D.K.; Robertson, L.B.; Tempest, H.G.; Vignal, A.; Fillon, V.; Crooijmans, R.P.; Groenen, M.A.; Deryusheva, S.; Gaginskaya, E.; Carré, W.; et al. Whole genome comparative studies between chicken and turkey and their implications for avian genome evolution. BMC Genom. 2008, 9, 168.
- Skinner, B.M.; Robertson, L.B.; Tempest, H.G.; Langley, E.J.; Ioannou, D.; Fowler, K.E.; Crooijmans, R.P.; Hall, A.D.; Griffin, D.K.; Völker, M. Comparative genomics in chicken and Pekin duck using FISH mapping and microarray analysis. BMC Genom. 2009, 10, 357.
- Fillon, V.; Vignoles, M.; Crooijmans, R.P.; Groenen, M.A.; Zoorob, R.; Vignal, A. FISH mapping of 57 BAC clones reveals strong conservation of synteny between Galliformes and Anseriformes. Anim. Genet. 2007, 38, 303–307.
- Christidis, L. Aves. In Animal Cytogenetics. Volume 4: Chordata 3 B.; John, B., Kayano, H., Levan, A., Eds.; Gebrüder Borntraeger: Berlin, Germany, 1990.
- Bloom, S.E. A current list of chromosome numbers and variations for species of the avian subclass Carinatae. J. Hered. 1969, 60, 217–220.
- Ray-Chaudhuri, R. Cytotaxonomy and chromosome evolution in birds. In Cytotaxonomy and Vertebrate Evolution; Chiarelli, A.B., Capanna, E., Eds.; Academic Press: New York, NY, USA, 1973; pp. 425–483.
- Shields, G.F. Comparative avian cytogenetics: A review. Condor 1982, 84, 45–58.
- de Boer, L.E.M. New developments in vertebrate cytotaxonomy VIII. A current list of references on avian karyology. Genetica 1984, 65, 3–6.
- Capanna, E.; Civitelli, M.; Martinico, E.I. I cromosomi degli uccelli. Citotassonomia ed evoluzione cariotipica. Avocetta 1987, 11, 101–143.
- Ellegren, H. Evolutionary stasis: The stable chromosomes of birds. Trends Ecol. Evol. 2010, 25, 283–291.
- Nie, W.; O’Brien, P.C.; Ng, B.L.; Fu, B.; Volobouev, V.; Carter, N.P.; Ferguson-Smith, M.A.; Yang, F. Avian comparative genomics: Reciprocal chromosome painting between domestic chicken (Gallus gallus) and the stone curlew (Burhinus oedicnemus, Charadriiformes)—An atypical species with low diploid number. Chromosome Res. 2009, 17, 99–113.
- Bian, X.; Li, Q. Studies on The Karyotypes of Birds V. The 20 species of Climber birds. (Aves). Zool. Res. 1989, 10, 309–317.
- Castro, M.S.; Recco-Pimentel, S.M.; Rocha, G.T. Karyotypic characterization of Ramphastidae (Piciformes, Aves). Genet. Mol. Biol. 2002, 25, 147–150.
- McQueen, H.A.; Siriaco, G.; Bird, A.P. Chicken microchromosomes are hyperacetylated, early replicating, and gene rich. Genome Res. 1998, 8, 621–630.
- Smith, J.; Paton, I.R.; Bruley, C.K.; Windsor, D.; Burke, D.; Ponce de Leon, F.A.; Burt, D.W. Integration of the genetic and physical maps of the chicken macrochromosomes. Anim. Genet. 2000, 31, 20–27.
- Habermann, F.A.; Cremer, M.; Walter, J.; Kreth, G.; von Hase, J.; Bauer, K.; Wienberg, J.; Cremer, C.; Cremer, T.; Solovei, I. Arrangements of macro- and microchromosomes in chicken cells. Chromosome Res. 2001, 9, 569–584.
- Burt, D.W. Origin and evolution of avian microchromosomes. Cytogenet. Genome Res. 2002, 96, 97–112.
- Backström, N.; Forstmeier, W.; Schielzeth, H.; Mellenius, H.; Nam, K.; Bolund, E.; Webster, M.T.; Ost, T.; Schneider, M.; Kempenaers, B.; et al. The recombination landscape of the zebra finch Taeniopygia guttata genome. Genome Res. 2010, 20, 485–495.
- Schield, D.R.; Card, D.C.; Hales, N.R.; Perry, B.W.; Pasquesi, G.M.; Blackmon, H.; Adams, R.H.; Corbin, A.B.; Smith, C.F.; Ramesh, B.; et al. The origins and evolution of chromosomes, dosage compensation, and mechanisms underlying venom regulation in snakes. Genome Res. 2019, 29, 590–601.
- Schield, D.R.; Pasquesi, G.I.M.; Perry, B.W.; Adams, R.H.; Nikolakis, Z.L.; Westfall, A.K.; Orton, R.W.; Meik, J.M.; Mackessy, S.P.; Castoe, T.A. Snake recombination landscapes are concentrated in functional regions despite PRDM9. Mol. Biol. Evol. 2020, 37, 1272–1294.
- Nakatani, Y.; Takeda, H.; Kohara, Y.; Morishita, S. Reconstruction of the vertebrate ancestral genome reveals dynamic genome reorganization in early vertebrates. Genome Res. 2007, 17, 1254–1265.
- Graves, J.A.M.; Shetty, S. Sex from W to Z: Evolution of vertebrate sex chromosomes and sex determining genes. J. Exp. Zool. 2001, 290, 449–462.
- Wang, Z.; Zhang, J.; Yang, W.; An, N.; Zhang, P.; Zhang, G.; Zhou, Q. Temporal genomic evolution of bird sex chromosomes. BMC Evol. Biol. 2014, 14, 250.
- Schmid, M.; Enderle, E.; Schindler, D.; Schemp, W. Chromosome banding and DNA replication patterns in bird karyotypes. Cytogenet. Cell Genet. 1989, 52, 139–146.
- Solari, A.J. Sex Chromosomes and Sex Determination in Vertebrates; CRC Press: Boca Raton, FL, USA, 1993.
- Nanda, I.; Schmid, M. Conservation of avian Z chromosomes as revealed by comparative mapping of the Z-linked aldolase B gene. Cytogenet. Genome Res. 2002, 96, 176–178.
- Schartl, M.; Schmid, M.; Nanda, I. Dynamics of vertebrate sex chromosome evolution: From equal size to giants and dwarfs. Chromosoma 2016, 125, 553–571.
- Shetty, S.; Griffin, D.K.; Graves, J.A.M. Comparative painting reveals strong chromosome homology over 80 million years of bird evolution. Chromosome Res. 1999, 7, 289–295.
- Takagi, N.; Itoh, M.; Sasaki, M. Chromosome studies in four species of Ratitae (Aves). Chromosoma 1972, 36, 281–291.
- Ansari, H.A.; Takagi, N.; Sasaki, M. Morphological differentiation of sex chromosomes in three species of ratite birds. Cytogenet. Cell Genet. 1988, 47, 185–188.
- Liu, J.; Wang, Z.; Li, J.; Xu, L.; Liu, J.; Feng, S.; Guo, C.; Chen, S.; Ren, Z.; Rao, J.; et al. A new emu genome illuminates the evolution of genome configuration and nuclear architecture of avian chromosomes. Genome Res. 2021, 31, 497–511.
- Deakin, J.E.; Ezaz, T. Tracing the evolution of amniote chromosomes. Chromosoma 2014, 123, 201–216.
- Nanda, I.; Shan, Z.; Schartl, M.; Burt, D.W.; Koehler, M.; Nothwang, H.; Grützner, F.; Paton, I.R.; Windsor, D.; Dunn, I.; et al. 300 million years of conserved synteny between chicken Z and human chromosome 9. Nat. Genet. 1999, 21, 258–259.
- Bellott, D.W.; Skaletsky, H.; Pyntikova, T.; Mardis, E.R.; Graves, T.; Kremitzki, C.; Brown, L.G.; Rozen, S.; Warren, W.C.; Wilson, R.K.; et al. Convergent evolution of chicken Z and human X chromosomes by expansion and gene acquisition. Nature 2010, 466, 612–616.
- Ross, M.T.; Grafham, D.V.; Coffey, A.J.; Scherer, S.; McLay, K.; Muzny, D.; Platzer, M.; Howell, G.R.; Burrows, C.; Bird, C.P.; et al. The DNA sequence of the human X chromosome. Nature 2005, 434, 325–337.
- Graves, J.A. Avian sex, sex chromosomes, and dosage compensation in the age of genomics. Chromosome Res. 2014, 22, 45–57.
- Smith, C.A.; Roeszler, K.N.; Ohnesorg, T.; Cummins, D.M.; Farlie, P.G.; Doran, T.J.; Sinclair, A.H. The avian Z-linked gene DMRT1 is required for male sex determination in the chicken. Nature 2009, 461, 267–271.
- Chen, N.; Bellott, D.W.; Page, D.C.; Clark, A.G. Identification of avian W-linked contigs by short-read sequencing. BMC Genom. 2012, 14, 183.
- Gunski, R.J.; Cañedo, A.D.; Garnero, A.D.V.; Ledesma, M.A.; Coria, N.; Montalti, D.; Degrandi, T.M. Multiple sex chromosome system in penguins (Pygoscelis, Spheniscidae). Comp. Cytogenet. 2017, 11, 541–552.
- Sigeman, H.; Ponnikas, S.; Hansson, B. Whole-genome analysis across 10 songbird families within Sylvioidea reveals a novel autosome–sex chromosome fusion. Biol. Lett. 2020, 16, 20200082.
- Huang, Z.; Furo, I.O.; Liu, J.; Peona, V.; Gomes, A.J.B.; Cen, W.; Huang, H.; Zhang, Y.; Chen, D.; Xue, T.; et al. Recurrent chromosome reshuffling and the evolution of neo-sex chromosomes in parrots. Nat. Commun. 2022, 13, 944.
- Kretschmer, R.; Gunski, R.J.; Garnero, A.d.V.; de Freitas, T.R.O.; Toma, G.A.; Cioffi, M.d.B.; Oliveira, E.H.C.d.; O’Connor, R.E.; Griffin, D.K. Chromosomal analysis in Crotophaga ani (Aves, Cuculiformes) reveals extensive genomic reorganization and an unusual Z-autosome Robertsonian translocation. Cells 2021, 10, 4.
- O’Connor, R.E.; Kiazim, L.; Skinner, B.; Fonseka, G.; Joseph, S.; Jennings, R.; Larkin, D.M.; Griffin, D.K. Patterns of microchromosome organization remain highly conserved throughout avian evolution. Chromosoma 2019, 128, 21–29.
- Ferguson-Smith, M.A.; Trifonov, V. Mammalian karyotype evolution. Nat. Rev. Genet. 2007, 8, 950–962.
- Graphodatsky, A.S.; Trifonov, V.A.; Stanyon, R. The genome diversity and karyotype evolution of mammals. Mol. Cytogenet. 2011, 4, 22.
- Damas, J.; Corbo, M.; Lewin, H.A. Vertebrate Chromosome Evolution. Annu. Rev. Anim. Biosci. 2021, 9, 1–27.
- Kretschmer, R.; Gunski, R.J.; Garnero, A.D.V.; Furo, I.O.; O’Brien, P.C.M.; Ferguson-Smith, M.A.; de Oliveira, E.H.C. Molecular cytogenetic characterization of multiple intrachromosomal rearrangements in two representatives of the genus Turdus (Turdidae, Passeriformes). PLoS ONE 2014, 9, e103338.
- Kretschmer, R.; Furo, I.O.; Gunski, R.J.; Garnero, A.D.V.; Pereira, J.C.; O’Brien, P.C.M.; Ferguson-Smith, M.A.; de Oliveira, E.H.C.; de Freitas, T.R.O. Comparative chromosome painting in Columbidae (Columbiformes) reinforces divergence in Passerea and Columbea. Chromosome Res. 2018, 26, 211–223.
- Kiazim, L.G.; O’Connor, R.E.; Larkin, D.M.; Romanov, M.N.; Narushin, V.G.; Brazhnik, E.A.; Griffin, D.K. Comparative mapping of the macrochromosomes of eight avian species provides further insight into their phylogenetic relationships and avian karyotype evolution. Cells 2021, 10, 362.
- Choudhuri, J.V.; Schleiermacher, C.; Kurtz, S.; Giegerich, R. GenAlyzer: Interactive visualization of sequence similarities between entire genomes. Bioinformatics 2004, 20, 1964–1965.
- Rao, M.; Morisson, M.; Faraut, T.; Bardes, S.; Fève, K.; Labarthe, E.; Fillon, V.; Huang, Y.; Li, N.; Vignal, A. A duck RH panel and its potential for assisting NGS genome assembly. BMC Genom. 2012, 13, 513.
- International Chicken Genome Sequencing Consortium. Sequence and comparative analysis of the chicken genome provide unique perspectives on vertebrate evolution. Nature 2004, 432, 695–716.
- Griffin, D.K.; Masabanda, J.S. Microchromosomal probes derived from individual chromosome microdissection were not successfully sequenced and since degraded in the freezer. 2006; unpublished results.
- Damas, J.; O’Connor, R.; Farré, M.; Lenis, V.P.E.; Martell, H.J.; Mandawala, A.; Fowler, K.; Joseph, S.; Swain, M.T.; Griffin, D.K.; et al. Upgrading short-read animal genome assemblies to chromosome level using comparative genomics and a universal probe set. Genome Res. 2017, 27, 875–884.
- Huang, Z.; Xu, Z.; Bai, H.; Huang, Y.; Kang, N.; Ding, X.; Liu, J.; Luo, H.; Yang, C.; Chen, W.; et al. Evolutionary analysis of a complete chicken genome. Proc. Natl. Acad. Sci. USA 2023, 120, e2216641120.
- Matsuda, Y.; Nishida-Umehara, C.; Tarui, H.; Kuroiwa, A.; Yamada, K.; Isobe, T.; Ando, J.; Fujiwara, A.; Hirao, Y.; Nishimura, O.; et al. Highly conserved linkage homology between birds and turtles: Bird and turtle chromosomes are precise counterparts of each other. Chromosome Res. 2005, 13, 601–615.
- Kasai, F.; O’Brien, P.C.; Ferguson-Smith, M.A. Reassessment of genome size in turtle and crocodile based on chromosome measurement by flow karyotyping: Close similarity to chicken. Biol. Lett. 2012, 8, 631–635.
- Hansmann, T.; Nanda, I.; Volobouev, V.; Yang, F.; Schartl, M.; Haaf, T.; Schmid, M. Cross-species chromosome painting corroborates microchromosome fusion during karyotype evolution of birds. Cytogenet. Genome Res. 2009, 126, 281–304.
- Dodgson, J.B.; Delany, M.E.; Cheng, H.H. Poultry genome sequences: Progress and outstanding challenges. Cytogenet. Genome Res. 2011, 134, 19–26.
- O’Connor, R.E.; Farré, M.; Joseph, S.; Damas, J.; Kiazim, L.; Jennings, R.; Bennett, S.; Slack, E.A.; Allanson, E.; Larkin, D.M.; et al. Chromosome-level assembly reveals extensive rearrangement in saker falcon and budgerigar, but not ostrich, genomes. Genome Biol. 2018, 19, 171.
- Joseph, S.; O’Connor, R.E.; Al Mutery, A.F.; Watson, M.; Larkin, D.M.; Griffin, D.K. Chromosome level genome assembly and comparative genomics between three Falcon species reveals an unusual pattern of genome organisation. Diversity 2018, 10, 113.
- Cheng, Y.; Burt, D.W. Chicken genomics. Int. J. Dev. Biol. 2018, 62, 265–271.
- Kretschmer, R.; de Souza, M.S.; Furo, I.d.O.; Romanov, M.N.; Gunski, R.J.; Garnero, A.d.V.; de Freitas, T.R.O.; de Oliveira, E.H.C.; O’Connor, R.E.; Griffin, D.K. Interspecies chromosome mapping in Caprimulgiformes, Piciformes, Suliformes, and Trogoniformes (Aves): Cytogenomic insight into microchromosome organization and karyotype evolution in birds. Cells 2021, 10, 826.
- Furo, I.O.; Kretschmer, R.; O’Brien, P.C.M.; Pereira, J.; Garnero, A.D.V.; Gunski, R.J.; O’Connor, R.E.; Griffin, D.K.; Gomes, A.J.B.; Ferguson-Smith, M.A.; et al. Chromosomal evolution in the phylogenetic context: A remarkable karyotype reorganization in neotropical parrot Myiopsitta monachus (Psittacidae). Front. Genet. 2020, 11, 721.
- Nanda, I.; Karl, E.; Griffin, D.K.; Schartl, M.; Schmid, M. Chromosome repatterning in three representative parrots (Psittaciformes) inferred from comparative chromosome painting. Cytogenet. Genome Res. 2007, 117, 43–53.
- Seligmann, I.C.A.; Furo, I.O.; dos Santos, M.S.; Tagliarini, M.M.; Araujo, C.C.D.; O’Brien, P.C.M.; Ferguson-Smith, M.A.; de Oliveira, E.H.C. Comparative chromosome painting in two Brazilian stork species with different diploid numbers. Cytogenet. Genome Res. 2019, 159, 32–38.
- Wang, J.; Su, W.; Hu, Y.; Li, S.; O’Brien, P.C.M.; Ferguson-Smith, M.A.; Yang, F.; Nie, W. Comparative chromosome maps between the stone curlew and three ciconiiform species (the grey heron, little egret and crested ibis). BMC Ecol. Evol. 2022, 22, 23.
- Seligmann, I.C.A.; Furo, I.O.; dos Santos, M.S.; Gunski, R.J.; Garnero, A.D.V.; Silva, F.A.O.; O’Brien, P.C.M.; Ferguson-Smith, M.A.; Kretschmer, R.; de Oliveira, E.H.C. Comparative chromosome painting in three Pelecaniformes species (Aves): Exploring the role of macro and microchromosome fusions in karyotypic evolution. PLoS ONE 2023, 18, e0294776.
- Kretschmer, R.; Franz, I.; de Souza, M.S.; Garnero, A.D.V.; Gunski, R.J.; de Oliveira, E.H.C.; O’Connor, R.E.; Griffin, D.K.; de Freitas, T.R.O. Cytogenetic evidence clarifies the phylogeny of the family Rhynchocyclidae (Aves: Passeriformes). Cells 2021, 10, 2650.
- Pala, I.; Hasselquist, D.; Bensch, S.; Hansson, B. Patterns of molecular evolution of an avian neo-sex chromosome. Mol. Biol. Evol. 2012, 29, 3741–3754.
- Pala, I.; Naurin, S.; Stervander, M.; Hasselquist, D.; Bensch, S.; Hansson, B. Evidence of a neo-sex chromosome in birds. Heredity 2012, 108, 264–272.
- Sigeman, H.; Ponnikas, S.; Chauhan, P.; Dierickx, E.; Brooke, M.d.L.; Hansson, B. Repeated sex chromosome evolution in vertebrates supported by expanded avian sex chromosomes. Proc. R. Soc. B 2019, 286, 20192051.
- Ellegren, H. The evolutionary genomics of birds. Annu. Rev. Ecol. Evol. Syst. 2013, 44, 239–259.
- Romanov, M.N.; Farré, M.; Lithgow, P.E.; Fowler, K.E.; Skinner, B.M.; O’Connor, R.; Fonseka, G.; Backström, N.; Matsuda, Y.; Nishida, C.; et al. Reconstruction of gross avian genome structure, organization and evolution suggests that the chicken lineage most closely resembles the dinosaur avian ancestor. BMC Genom. 2014, 15, 1060.
- Maslova, A.; Zlotina, A.; Kosyakova, N.; Sidorova, M.; Krasikova, A. Three-dimensional architecture of tandem repeats in chicken interphase nucleus. Chromosome Res. 2015, 23, 625–639.
- Perry, B.W.; Schield, D.R.; Adams, R.H.; Castoe, T.A. Microchromosomes exhibit distinct features of vertebrate chromosome structure and function with underappreciated ramifications for genome evolution. Mol. Biol. Evol. 2021, 38, 904–910.
- Waters, P.D.; Patel, H.R.; Ruiz-Herrera, A.; Álvarez-González, L.; Lister, N.C.; Simakov, O.; Ezaz, T.; Kaur, P.; Frere, C.; Grützner, F.; et al. Microchromosomes are building blocks of bird, reptile, and mammal chromosomes. Proc. Natl. Acad. Sci. USA 2021, 118, e2112494118.
- Tanabe, H.; Müller, S.; Neusser, M.; von Hase, J.; Calcagno, E.; Cremer, M.; Solovei, I.; Cremer, C.; Cremer, T. Evolutionary conservation of chromosome territory arrangements in cell nuclei from higher primates. Proc. Natl. Acad. Sci. USA 2002, 99, 4424–4429.
- Tanabe, H.; Küpper, K.; Ishida, T.; Neusser, M.; Mizusawa, H. Inter- and intra-specific gene-density-correlated radial chromosome territory arrangements are conserved in Old World monkeys. Cytogenet. Genome Res. 2005, 108, 255–261.
- Srikulnath, K.; Ahmad, S.F.; Singchat, W.; Panthum, T. Why Do Some Vertebrates Have Microchromosomes? Cells 2021, 10, 2182.
- Mengden, G.A.; Stock, A.D. Chromosomal evolution in Serpentes: A comparison of G and C chromosome banding patterns of some Colubrid and Boid genera. Chromosoma 1980, 79, 53–64.
- Olmo, E. Trends in the evolution of reptilian chromosomes. Integr. Comp. Biol. 2008, 48, 486–493.
- Srikulnath, K.; Thapana, W.; Muangmai, N. Role of chromosome changes in Crocodylus evolution and diversity. Genom. Inform. 2015, 13, 102.
- Cohen, M.M.; Gans, C. The chromosomes of the order Crocodilia. Cytogenetics 1970, 9, 81–105.
- Pokorná, M.; Giovannotti, M.; Kratochvíl, L.; Caputo, V.; Olmo, E.; Ferguson-Smith, M.A.; Rens, W. Conservation of chromosomes syntenic with avian autosomes in squamate reptiles revealed by comparative chromosome painting. Chromosoma 2012, 121, 409–418.
- King, M. Species Evolution: The Role of Chromosome Change; Cambridge University Press: Cambridge, UK, 1995.
- Gregory, T.R. A bird’s-eye view of the C-value enigma: Genome size, cell size, and metabolic rate in the class aves. Evolution 2002, 56, 121–130.
- Andrews, C.B.; Mackenzie, S.A.; Gregory, T.R. Genome size and wing parameters in passerine birds. Proc. Biol. Sci. 2009, 276, 55–61.
- Janes, D.E.; Organ, C.L.; Fujita, M.K.; Shedlock, A.M.; Edwards, S.V. Genome evolution in Reptilia, the sister group of mammals. Annu. Rev. Genomics Hum. Genet. 2010, 11, 239–264.
- Organ, C.L.; Shedlock, A.M.; Meade, A.; Pagel, M.; Edwards, S.V. Origin of avian genome size and structure in non-avian dinosaurs. Nature 2007, 446, 180–184.
- Organ, C.L.; Shedlock, A.M. Palaeogenomics of pterosaurs and the evolution of small genome size in flying vertebrates. Biol. Lett. 2009, 5, 47–50.
- Hughes, A.L.; Hughes, M.K. Small genomes for better flyers. Nature 1995, 377, 391.
- Garner, J.P.; Taylor, G.K.; Thomas, A.L.R. On the origins of birds: The sequence of character acquisition in the evolution of avian flight. Proc. Biol. Sci. 1999, 266, 1259.
This entry is offline, you can click here to edit this entry!
