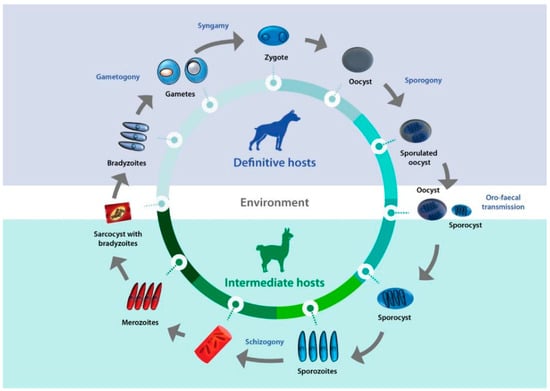
Sarcocystis spp. are coccidian protozoans belonging to the Apicomplexa phylum. As with other members of this phylum, they are obligate intracellular parasites with complex cellular machinery for the invasion of host cells. Sarcocystis spp. display dixenous life cycles, involving a predator and a prey as definitive and intermediate hosts, respectively. Specifically, these parasites develop sarcocysts in the tissues of their intermediate hosts, ranging in size from microscopic to visible to the naked eye, depending on the species. When definitive hosts consume sarcocysts, infective forms are produced in the digestive system and discharged into the environment via feces. This review article focuses on the current knowledge on biological features, diagnostic methods, pathogenicity, epidemiology and host-pathogen interactions of Sarcocystis spp. that infect camelids from the Old and New World.
1. Introduction
Sarcocystosis is a parasitic infection caused by different species of protozoans belonging to the
Sarcocystis genus. With over 200 described species, different
Sarcocystis spp. infect mammals, including humans, as well as birds and reptiles. Their life cycles involve a predator and a prey that serve as definitive and intermediate hosts, respectively. Typical of the intermediate host phase of
Sarcocystis is the formation of sarcocysts—wall-surrounded capsules in which the parasites divide asexually—which may be micro or macroscopic, depending on the species [
1].
Sarcocystis complex life cycles alternate between invading, dividing, and sexual stages. After a predator ingests the meat of a prey containing sarcocysts, bradyzoites—the infective stage borne in these structures—invade the goblet cells of the predator’s intestine. Micro- and macrogametes are formed, and gamete fusion leads to the formation of an oocyst which, after being excreted with the feces into the environment, sporulates to form four sporozoites. Intermediate hosts ingest oocysts when grazing or drinking contaminated pasture or water, and enzymes in their intestine lead to the liberation of sporozoites. Individual sporozoites can also be found in stools due to occasional breakage of the thin oocyst wall, and can contaminate water and pastures, which leads to direct ingestion of sporozoites by the intermediate host. Once in the intestinal lumen, sporozoites invade the endothelial cells of mesenteric lymph node arteries, where they reproduce asexually by schizogony, forming schizonts with lobed nuclei that have the appearance of a rosette. Merozoites eventually bud off and are released into the bloodstream, disseminating the infection in the intermediate host. They can be found free or inside mononuclear cells, where they undergo binary division by endodyogeny. When merozoites invade the endothelial cells of downstream arterioles, capillaries, and veins, a new cycle of schizogony is initiated. In turn, invasion of a myocyte or a nervous cell by a merozoite leads to the formation of sarcocysts, the final stage in the intermediate host. The parasite remains inside a parasitophorous vacuole (PV), and the parasitophorous vacuolar membrane (PMV) together with parasite secretions form a wall that provides a safe microenvironment for parasite multiplication. Depending on the
Sarcocystis species, final-destination cells can be myocytes of skeletal or cardiac muscles, or neural cells. In the sarcocysts, merozoites transform into metrocytes, which rapidly divide by endodyogeny and eventually turn into infective bradyzoites [
1,
2,
3] (
Figure 1).
Figure 1. Life cycle of Sarcocystis. A typical life cycle of Sarcocystis species is shown, exemplified with a llama and a dog as intermediate and definitive hosts, respectively.
Most
Sarcocystis spp. that infect livestock have a worldwide distribution and, in general, occur with high prevalence in both industrialized and developing countries [
3]. The economic burden of
Sarcocystis infections of livestock is related to abortions, low meat and/or milk yield, poor body growth, and outbreaks of clinical sarcocystosis that can be fatal. Additionally, carcasses destined for human consumption can be condemned by sanitary authorities when abundant macroscopic
Sarcocystis spp. sarcocysts or
Sarcocystis spp.-associated lesions due to eosinophilic myositis are encountered. Eosinophilic myositis is an inflammatory condition of striated muscles that leads to necrosis of the affected areas which has been described in cattle and some other mammals [
1,
4,
5]. For most
Sarcocystis species, especially those infecting wild animals, their effect on host fitness is unknown.
Camelids are Artiodactyla mammals grouped in the Camelidae family, the only extant family of the Tylopoda suborder (from the Greek: “feet with cushions”). They regurgitate and rechew food but are not ruminants and are differentiated from the latter by several anatomical features, including their stomach with three compartments, lack of horns, and the presence of real canine teeth and plantar cushions, as well as other physiological and behavioral features. Importantly, camelids differ from ruminants in their susceptibility to microbial and parasitic diseases [
6].
Old World camelids (OWCs) belong to the genus
Camelus, which comprises three extant species:
C. dromedarius (one-humped camel or dromedary),
C. bactrianus (two-humped or Bactrian camel), both of which have been domesticated, and the wild and highly endangered
C. ferus (two humps). The population size of OWCs has been estimated to be at least 35.5 million heads, of which 95% are dromedaries [
7]. South American camelids (SACs) comprise four species: the wild
Lama guanicoe (guanaco) and
Vicugna vicugna (vicuña), and the domesticated
Lama glama (llama) and
Vicugna pacos (alpaca), with an overall estimated population of 10 million heads [
8]. Notably, llamas and alpacas have been introduced to farms in some European countries, South Africa, and Australia and, thus, their geographic distribution and numbers of heads are considerably larger than those mentioned here [
7,
8].
Both OWCs and SACs are adapted to harsh environmental conditions, including extreme temperatures, intense solar radiation, water scarcity, and poor pastures. Under such rough settings, most other livestock species are either unable to thrive or show a significant decline in production. Camelids thus constitute an attractive livestock choice in a scenario of climate change, shortage of water, and reduction in agricultural areas [
9].
Camelids have a long history of association with humans. Human groups who lived as gatherers and hunters must have found in camels a good source of food and hides, and through their domestication some 3 to 7 thousand years ago (kya), they became important suppliers of vital goods to ancient civilizations in the Old and New Worlds [
10,
11]. Currently, camelids continue to be an important asset to a considerable number of human populations, and among other benefits, their meat is a valuable source of animal protein and an attractive product for the gourmet cuisine [
12,
13,
14].
Both OWCs and SACs act as intermediate hosts for some
Sarcocystis spp., sometimes with a relevant negative impact on local economies [
15,
16,
17,
18].
2. Sarcocystis Infecting OWCs and SACs
Four
Sarcocystis spp. have so far been described to infect camelids:
S. cameli and
S. ippeni for OWCs, and
S. aucheniae and
S. masoni for SACs [
3,
17,
19].
The first observation of
Sarcocystis infections in OWCs was carried out in Egypt by Mason (1910), who reported macroscopic sarcocysts in the muscles of camels and used the name
S. cameli to refer to the etiological agent. Later, several additional case reports of
Sarcocystis species infecting OWCs appeared in the literature, which were designated as
S. ippeni,
S. camelicanis,
S. camelocanis, and
S. miescheri, depending on their sarcocyst wall or oocyst features. A taxonomic revision of OWCs sarcocystosis led to the acceptance of
S. cameli and
S. ippeni as valid species, while
S. camelicanis,
S. camelocanis, and
S. miescheri were considered synonymous with
S. cameli. Importantly, the vast majority of
Sarcocystis reports are from dromedary camels [
16,
19,
20].
In the case of SAC, the first description of a macroscopic sarcocyst in a llama took place in 1913, and the corresponding parasite was named
S. aucheniae [
21]. Later, the names
S. tilopodi and
S. guanicoecanis were used for parasites forming sarcocysts in guanaco [
22,
23]. A
Sarcocystis species forming microscopic cysts in SACs was initially named
S. lamacanis [
24,
25]. Electron microscopy and molecular studies established
S. aucheniae as the only species forming macroscopic cysts in llamas, alpacas, and guanacos, while the species forming microscopic cysts was redescribed as
S. masoni in honor of Dr Eugene Mason. Thus, the names
S. tilopodi,
S. guanicoecanis, and
S. lamacanis are currently considered invalid [
3,
17,
26].
In different
Sarcocystis spp., sarcocysts vary in shape (globular, filamentous, fusiform), size, and other characteristics, such as the presence or absence of internal partitions and variations in their wall ultrastructure [
2,
3]. In the case of
Sarcocystis spp. that infect camelids, both
S. aucheniae and
S. cameli generate macroscopic sarcocysts that are visible to the naked eye (oval, 5–20 mm × 2 mm, and fusiform, 1.5–5 mm × 0.2–0.4 mm, respectively). Additionally, microscopic cysts of
S. cameli (700 × 100 µm) are commonly found in camel tissues. In turn, only microcysts were described for
S. masoni (fusiform, 800 × 95 µm) and
S. ippeni (globular, 100–120 × 50–100 µm) [
3,
17,
19] (
Table 1).
Table 1. Characteristics of sarcocysts produced by camelid-infecting Sarcocystis sp.
In all
Sarcocystis spp., the sarcocyst wall essentially consists of the PVM covering a granular, electron-dense layer from which septa can arise. When present, septa cross the cyst, separating its cavity into compartments, where metrocytes and bradyzoites are found. The number of parasites contained in a sarcocyst varies with the species and the stage of maturation: young cysts as small as 5 μm in diameter might contain only two parasites, while a mature macroscopic cyst can contain 20 million, as has been observed for
S. aucheniae [
3,
26].
The sarcocyst wall can remain relatively simple in some species and, in others, form projections (villar protrusions) of different sizes and shapes that bulge outwardly and can contain microfilaments, microtubules, electron-dense bodies, minute granules, and small vesicles [
1,
3]. At least 82 ultrastructural types of cyst wall have been described for different
Sarcocystis spp. [
3]. Notably, the cyst walls of both
S. cameli and
S. masoni have a common “9j” conformation, characterized by the presence of upright finger-like villar protrusions with knob-like structures arising from the PVM, in which microtubules can be observed [
3,
17].
S. aucheniae presents a ‘type 21’ sarcocyst wall ultrastructure, with highly branched cauliflower-like villar protrusions, similar to that of
S. gigantea [
1,
3,
17]. Finally,
S. ippeni has a characteristic ‘type 32’ sarcocyst wall structure. This type of ultrastructure is characterized by thorn-like villar protrusions with microtubules radiating into the granular layer and has not been previously described in any other
Sarcocystis species [
3] (
Table 1).
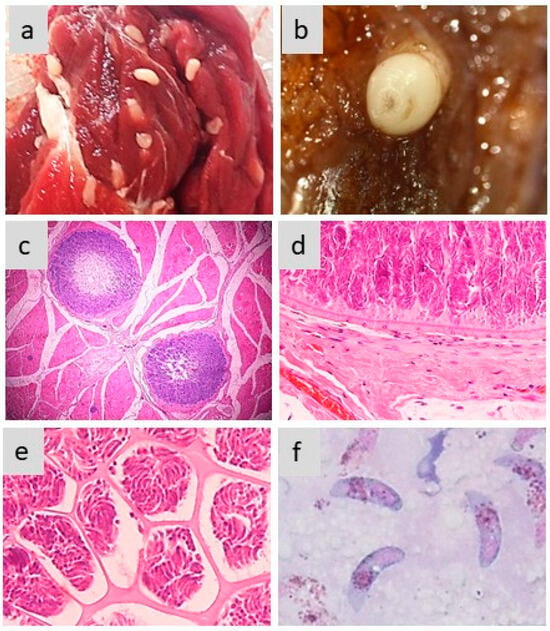
Figure 2 exemplifies the different aspects of sarcocysts produced by S. aucheniae in the skeletal muscles of llamas and alpacas.
Figure 2. Morphology of sarcocysts and bradyzoites in S. aucheniae. (a,b) Macroscopic sarcocysts in llama (a) and alpaca (b) skeletal muscle; (c) hematoxylin eosin-dyed cross-section of alpaca skeletal muscle with two macroscopic sarcocysts, in which zoites are located to the periphery and the center is empty (100×); (d,e) details of hematoxylin eosin-dyed section of a macroscopic sarcocyst showing the morphology of the cell wall (d) and compartments with thousands of banana-shaped bradyzoites (e) (400×); (f) bradyzoites observed in a cyst stained with Giemsa (1000×). The photographs were obtained by S.N.W. and L.V.M.O.
This entry is adapted from the peer-reviewed paper 10.3390/pathogens13030196