Dysfunction of the neuroglia can have profound consequences on the blood–brain barrier (BBB). Studies have shown that the disruption of astrocytic–endothelial interaction can compromise the permeability of BBB and its effectiveness in selectively regulating the exchange of substances. Microglia have been recognized to have a significant role in the initiation of chronic pain and in its interactions with various nerve blockers and anesthetic agents. Microglia have a role in pain resolution via a pathway that involves Cannabinoid receptor type 2 activation and MAP kinase phosphorylation.
1. Introduction
The blood–brain barrier (BBB) plays a crucial role in protecting the central nervous system (CNS). The BBB is comprised of tightly sealed endothelial cells lining the blood vessels of the brain, which serve to strictly block the passage of undesired molecules into the brain. The role of the BBB is to safeguard what molecules, drugs, and nutrients are able to cross from the peripheral bloodstream into the brain tissue [
1]. The semipermeable nature of the BBB selectively allows the passage of nutrients and desired molecules into the brain tissue and prevents the passage of potentially harmful toxins, pathogens, or autoantibodies [
2].
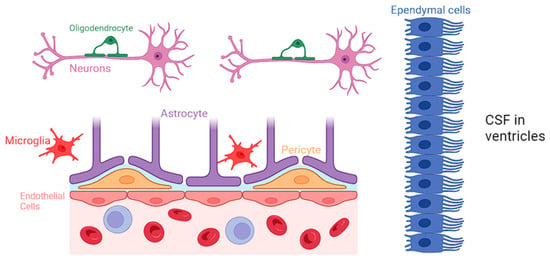
Neuroglia constitute a diverse group of cells within the CNS, comprising astrocytes, microglia, oligodendrocytes, and ependymal cells [
3]. While neurons are traditionally considered the primary functional units of the nervous system, neuroglia provide crucial support, maintaining the structural and functional integrity of the neural environment [
3]. A comprehensive representation of all the various types of neuroglial cells is presented in
Figure 1.
Figure 1. Biochemical specifics of the BBB and the specific role of each neuroglial cell.
Astrocytes are the most abundant type of neuroglia and are particularly noteworthy for their intricate association with the BBB. They closely interact with the endothelial cells of the CNS and regulate the permeability of BBB by releasing various signaling molecules, including growth factors and cytokines, and maintaining the tight junctions in the blood vessels [
4]. Astrocytes have a protrusion called “end-feet”, which allows them to sheath brain vasculature to maintain tight junctions and regulate blood flow [
5]. Moreover, astrocytes play a role in regulating the transport of nutrients and waste products across the BBB, contributing to the overall homeostasis of the neural environment [
6].
Microglia are traditionally known for their immune surveillance and response functions. However, recent research has shed light on the intricate involvement of microglia in BBB regulation [
7]. They actively participate in the modulation of tight junctions between endothelial cells, contributing to the selective permeability of the BBB. Studies have identified that following dysfunction of the BBB, microglia release factors such as transforming growth factor-beta (TGF-β) and interleukin-10 (IL-10), which have been shown to enhance the integrity of the BBB by promoting tight junction formation [
8,
9]. Microglia are also able to phagocytize cellular debris and pathogens that may be causing BBB disruption, and clearance of such materials can help return the BBB to a more normal state of functioning. Additionally, microglia can interact with astrocytes to promote the secretion of growth factors that can help heal damaged components of the BBB [
10,
11,
12].
Oligodendrocytes play a pivotal role in the CNS by producing myelin and facilitating rapid electrical signal transmission. While the primary function of oligodendrocytes lies in myelination, emerging research suggests their involvement in BBB regulation. Studies have demonstrated that oligodendrocytes are closely associated with endothelial cells of the BBB, and they may participate in the modulation of barrier permeability. The interactions between oligodendrocytes and endothelial cells at the BBB highlight the complexity of neuroglial contributions to the maintenance of a tightly regulated neural microenvironment [
13,
14].
Ependymal cells contribute to the functionality of BBB primarily through their role in cerebrospinal fluid (CSF) production and circulation but also via their involvement in modulating the BBB’s permeability. Recent studies have highlighted the expression of tight junction proteins in ependymal cells, indicating their potential contribution to the barrier function [
15]. Ependymal cells can also work along with other glial cells, influencing the overall regulation of brain homeostasis.
Furthermore, pericytes serve the BBB by promoting blood vessel formation, maintaining the BBB, regulating immune cell entry, and controlling blood flow [
16,
17]. Pericytes stimulate angiogenesis through the secretion of vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and angiopoietin-1, which can support the development of new capillaries. Additionally, pericytes control vasodilation and vasoconstriction, which allows them to control blood flow corresponding to the needs of the tissue. This helps tissue regenerate from damage and ensures the strength of the vasculature making up the BBB [
18,
19,
20].
Dysfunction of the neuroglia can have profound consequences on the BBB. Studies have shown that the disruption of astrocytic–endothelial interaction can compromise the permeability of BBB and its effectivity on selectively regulating the exchange of substances between blood and the brain [
7]. This compromised barrier function allows for the uncontrolled entry of potentially harmful molecules, including inflammatory mediators and toxins, into the brain parenchyma. This disruption can contribute to the development of neuroinflammatory diseases like multiple sclerosis (MS), neuromyelitis optica (NMO), and systemic lupus erythematosus (SLE) [
2]. Dysfunction of the BBB plays a particular role in autoimmune neurological disorders due to effector molecules of the peripheral immune system being able to enter the brain and stimulate an inflammatory response, leading to disruption of normal neural function [
21].
2. Pathophysiology of Pain
Pain is a nuanced and intricate experience, functioning as a major alarm system that signifies a potential threat or injury, which serves an adaptive role in protecting the body from harm. Yet, in certain cases, chronic pain can evolve into a maladaptive condition, causing significant personal and economic burdens [
22,
23,
24,
25,
26]. Thus, effective pain management becomes crucial in mitigating these challenges, as it not only improves the individual’s quality of life but also reduces the broader societal impact associated with healthcare costs, productivity loss, and emotional distress. However, the dynamics of pain involve a more complex interaction among biological, psychological, and emotional systems [
27], and the perception of pain varies significantly among individuals, highlighting the high degree of inter- and intra-patient variability [
28]. Recent advances in imaging modalities, such as functional magnetic resonance imaging (fMRI) and positron emission tomography (PET), have significantly enhanced our understanding of the central role played by the brain in the intricate processes of perceiving and modulating pain signals through the ascending and descending pathways.
These advances have also shed light on the interactions that macrophages have on our ability to feel pain. Many research studies have been conducted to analyze macrophages and their interactions with various nociceptors in our body. The Toll-like receptors that begin to circulate in our body after activation of nociceptors have been shown to activate macrophages and lengthen the pain that we feel. This is imperative to understanding when thinking about the differences between the ascending and descending pathways.
3. Introduction to Various Nerve Blockers Used in Anesthesia
The peripheral nerve blocks terminate pain signals the cerebral cortex receives from the spinal cord. Perioperative anesthetic nerve blocks can manage pain after procedures and reduce the need for postsurgical opioid consumption [
76] when administered along with general anesthesia or autonomously in less complex surgeries using ultrasound-guided techniques [
77]. Diagnostic nerve blocks in chronic pain can determine the anatomical source of pain signals and provide therapeutic utility [
78]. Nerve blocks can reduce inflammation and provide temporary pain relief for acute and chronic upper and lower extremity pain. Damage to a sympathetic nerve chain can be used as a target for sympathetic nerve blocks when autonomic function damage and sympathetically mediated pain (SMP) occurs. Stellate ganglion blocks further identify upper limb, head, and neck region nerve damage and block neural connections, improving the blood supply of the region and reducing adrenal hormone plasma concentration [
79]. To diagnose facet joints as a source of pain, placebo-controlled zygapophysial blocks can be a cost-effective alternative to lumbar medial branch neurotomy [
80]. The researchers have summarized several types of common nerve blocks depending on the injury, clinical indications, and side effects in
Table 1.
Table 1. Summary of the various types of common nerve blocks depending on the injury, clinical indications, and side effects.
|
Procedure
|
Mechanism
|
Indication
|
Side Effects
|
Reference
|
|
Celiac plexus block (CPB)
|
Targets visceral afferent pain fibers from the liver, gallbladder, omentum, pancreas, mesentery, and stomach to the mid-transverse colon
|
Pain secondary to pancreatic cancer, chronic pancreatitis, and intractable abdominal pain
|
Transient or persistent diarrhea, paraplegia (anterior spinal artery syndrome), postural hypotension, pneumothorax
|
[81,82,83]
|
|
Epidural nerve block
|
Injected anesthetic in the epidural space temporarily numbs spinal nerves, blocking pain signals from spinal cord levels
|
Surgical procedures: pelvic fractures, cesarean delivery, labor analgesia, hepatic, gastric, and colonic surgeries
Nonsurgical: myasthenia gravis, malignant hyperthermia, hyperreflexia
|
Hypotension, nausea, vomiting, post-puncture headache after dural perforation. [9] Incidence of transient paralysis is 0.1%; that of permanent paralysis is 0.02% [10]. Paresthesia with or without motor weakness, epidural hematoma, abscess, hypoalgesia of lower extremities
|
[84,85]
|
|
Genicular nerve block (GNB)
|
Anesthetizes sensory nerve terminal branches of genicular arteries or at the junction of the epiphysis and diaphysis of the femur and tibia, sparing motor function
|
Chronic knee osteoarthritis, post-operative knee pain, total knee arthroplasty, alternative to femoral, fascia iliaca, and adductor canal nerve blocks in knee injuries [11]
|
Leg muscle weakness, dizziness, and discomfort at injection site
|
[86,87,88]
|
|
Intercostal nerve block (ICNB)
|
Anesthetic injection to intercoastal nerves below each rib
|
Rib fracture neuralgia, thoracostomy analgesia, herpes zoster neuralgia, upper abdominal surgery, palliative cancer pain for rib and chest wall tumors
|
Self-limited bruising and soreness at the injection site. Serious: bleeding, infection, pneumothorax, nerve damage
|
[89,90]
|
|
Lumbar sympathetic nerve block
|
Disrupts the nerve supply from the preganglionic neurons exiting the spinal cord via the white rami of the ventral root of spinal nerves L1 to L4 and synapse at the lumbar sympathetic ganglion to the postganglionic neurons innervating the lower extremities
|
Sciatica, Complex Regional Pain Syndrome (CPRS), phantom limb pain, and lower limb painful ischemia
|
Flushing of skin, bleeding, bruising, soreness at the injection site, headache, and leg weakness on ipsilateral injection. Serious: infection, visceral injury, Horner’s syndrome
|
[91,92,93]
|
|
Occipital nerve block
|
C2 sensory neurons of the greater occipital nerve create a nociceptive pathway with the trigeminal nucleus caudalis, relieving compression and nerve irritation when targeted with an anesthetic
|
Occipital neuralgia, chronic intractable migraine, and cervicogenic and cluster headache treatment alternative in elderly and pregnant populations
|
Dizziness, vertigo, numbness, lightheadedness, vasovagal syncope, facial edema, and alopecia at injection if administered with steroid
|
[94,95]
|
|
Pudendal nerve block
|
Transcutaneous (perineal) or transvaginal approach targets the pudendal nerve trunk and its sensorimotor innervation
|
Pudendal neuralgia, obstetric (e.g., second stage of vaginal birth, vaginal repairs, hemorrhoidectomy), and urologic procedures (e.g.,transrectal ultrasound-guided prostate biopsy, transurethral prostatectomy)
|
Discomfort at the injection site, serious side effect of bladder and rectum structural injury, and pudendal artery puncture infection
|
[96,97]
|
|
Stellate ganglion block
|
Interrupts signals to the cervical sympathetic chain and postganglionic fibers for sympathetic innervation of upper limbs
|
CRPS of head and upper limbs, peripheral vascular disease, chronic post-surgical pain, postherpetic neuralgia, orofacial pain, scleroderma
|
Temporary pain, eyelid droopiness, fever, local blood aspiration, hematoma formation, spondylitis, and rare convulsions
|
[98,99]
|
|
Trigeminal nerve block
|
The ophthalmic (V1), maxillary (V2), and mandibular (V3) divisions and their corresponding nerves are blocked
|
Trigeminal neuralgia, pre-emptive analgesia in maxillofacial surgery
|
Difficulty chewing and swallowing and transient facial weakness and numbness
|
[100,101]
|
This entry is adapted from the peer-reviewed paper 10.3390/neuroglia5010002