Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Vaccination is undoubtedly the most cost-effective intervention to curb the HIV pandemic, both in the preventative and therapeutic setting. Based on the epidemiological evidence and experimental data indicating a protective role for anti-Tat immunity, in particular of anti-Tat Abs, the development of vaccines based on Tat was undertaken.
- HIV-1 Tat protein
- extracellular Tat protein
- HIV-1 infection
- HIV vaccine
- HIV preventative vaccine
1. Interaction of eTat with Host Receptors: A Way to Immune Dysregulation
HIV-1 infection is characterized by a generalized dysregulation of innate and adaptive immunity mediated by myeloid and lymphoid cells, respectively [1]. The molecules mentioned above and binding eTat are expressed mostly on myeloid and to a lesser extent lymphoid cells, and their engagement by eTat has been reported to alter their regulation and function, thus contributing to the sustained inflammation and immune dysregulation characterizing disease progression (Figure 1) [1]. The overall outcome is a dysregulated immune activation and suppression, driving on the one hand HIV dissemination and latency, on the other hand, immune deficiency and the occurrence of co-morbidities. Of note, this is further compounded by the reported dichotomous role Tat has on cell survival and death. In general, in infected cells (intracellular) Tat appears to promote cell survival by inducing Bcl2 and blocking Bim, whereas in uninfected cells eTat promotes apoptosis [2][3][4].
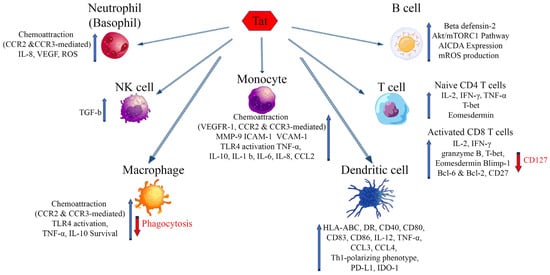
Figure 1. Effects of extracellular Tat on cells that are key players of the innate and adaptative immune response. Arrows indicate increase (blue) or decrease (red) of the markers shown. AICDA: Activation-induced cytidine deaminase; Bcl-2, -6: B-cell lymphoma 2, 6; Blimp-1: B lymphocyte-induced maturation protein-1; CCR2: C-C chemokine receptor type 2; CCR3: C-C chemokine receptor type 3; CCL-2, -3, -4: chemokine (C-C motif) ligand 2, 3, 4; HLA-ABC: Human leukocyte antigens A, B, C; HLA-DR: HLA class II cell surface receptor; ICAM-1: Intercellular Adhesion Molecule 1; IDO-1: Indoleamine 2,3-dioxygenase; IL: Interleukin; IFN-γ: Interferon gamma; MMP-9: Matrix metalloproteinase-9; mROS: mitochondrial reactive oxygen species; mTORC1: mammalian target of rapamycin complex 1; PD-L1: Programmed death-ligand 1; T-bet: T-box transcription factor TBX21; VEGF: Vascular endothelial growth factor; VEGFR-1: Vascular endothelial growth factor receptor 1; VCAM-1: Vascular cell adhesion protein 1.
2. Antibodies to Tat Are Associated with No or Delayed Progression to Disease
Anti-Tat antibodies (Abs) are infrequent in PLWH [5][6]. Of note, an inverse relationship between progression to disease (associated with p24 antigenemia, plasma VL and CD4 T cell loss) and anti-Tat seropositivity was noticed, suggesting a protective role of anti-Tat Ab [7][8][9][10]. Indeed, a significantly lower risk of progression to disease was observed in a cohort of 252 HIV-1 seroconverters followed for up to 14 years (median follow-up time: 7.2 years) [11]. Similarly, the time to cART initiation differed markedly according to the anti-Tat Ab serostatus in a subsequent prospective observational (OBS) study (NCT01029548) (Table 1) [12]. In fact, of the 61 asymptomatic individuals naive to cART and followed for up to 42 months (median follow-up time: 24 months), all of those seronegative for anti-Tat Abs had to start cART by 17 months, while none of the 20 (32.8%) subjects with a broad (all the three classes of immunoglobulins) and durable anti-Tat Ab response had to do so by the end of the study (Table 1) [12]. Further, the anti-Tat serostatus also affects the response to cART initiation. A faster (>3 times) and stronger (persistently undetectable VL) response to therapy was observed in anti-Tat Ab positive subjects as compared to anti-Tat Ab negative individuals (p < 0.0001, log-rank test (OBS-IFO) (Table 1) [13]. In individuals on long-term ART, anti-Tat immunity was associated with higher nadir CD4 T cell counts, long-lasting CD4 T cell recovery and the control of low-level viremia [14]. Interestingly, the anti-Tat Ab level was associated with the control of very-low-level viremia (≤40 copies/mL) whereas the occurrence of higher (>40 copies/mL) viral load was not affected by the anti-Tat serostatus (Table 1) [15]. It will be of interest to determine whether inducing or boosting anti-Tat immune responses improves virus control in individuals on long-term ART. Thus, strategies aimed at inducing Abs capable of neutralizing the biological activity of eTat should be pursued to halt the eTat-mediated maintenance of HIV-1 reservoirs and reduce inflammation and immune activation and dysregulation.
Table 1. Clinical benefits associated to the presence of anti-Tat antibodies.
| Study Code | Volunteers Number | Status | Results | Potential Clinical Benefit |
|---|---|---|---|---|
| ISS OBS T-003 (NCT01029548) [12] |
73 | naïve to cART | Stable CD4 T cell counts and contained viral load in anti-Tat Ab positive individuals throughout the study (3 years) Persistently anti-Tat Ab positive: no progression or therapy initiation throughout the study (3 years) Transiently Ab positive: therapy initiation after 30 months Anti-Tat Ab negative: therapy initiation after 17 months |
Prevention of progression |
| OBS-IFO [13] |
29 | starting cART | Faster and persistent virologic response to cART, in anti-Tat Ab positive as compared to anti-Tat Ab negative individuals | Improved time-to-response to therapy |
| ISS OBS T-002 (NCT01024556) [15] |
127 | on cART | CD4 T cell increase upon cART as compared to anti-Tat Ab neg individuals throughout the study (3 years) | Therapy intensification |
Table reproduced and modified with permission from ref. [14].
3. Strategies to Neutralize eTat in Preventative and Therapeutic Vaccine Approaches
Vaccination is undoubtedly the most cost-effective intervention to curb the HIV pandemic, both in the preventative and therapeutic setting. Based on the epidemiological evidence and experimental data indicating a protective role for anti-Tat immunity, in particular of anti-Tat Abs, the development of vaccines based on Tat was undertaken.
3.1. Pre-Clinical and Early (Phase I) Clinical Development
The Tat vaccine underwent an extensive (9 studies employing 112 Mauritian cynomolgus monkeys) preclinical evaluation in nonhuman primates [16][17][18][19]. Overall, Tat vaccination reduced infection acquisition and contained acute CD4 T cell loss in both acute and chronic infection [17].
Based on these results, preventative (ISS P-001, ClinicalTrials.gov Identifier: NCT00529698; ISS T-001) and therapeutic (ClinicalTrials.gov Identifier: NCT00505401), double-blind, placebo-controlled phase I trials with the biologically active Tat were conducted in Italy, meeting both primary (safety) and secondary (immunogenicity) endpoints (Table 2) [20][21]. The Tat vaccine was safe and it did not induce virus replication in the HIV-infected volunteers, as indicated by the CD4 T cell counts preservation and the absence of significant plasma viremia rebounds. Antibody response to Tat lasted up to 5 years after vaccination, as determined in the long-term follow-up (ISS OBS P-001, ClinicalTrials.gov Identifier: NCT01024764) [20][21].
A phase I preventative trial with Tat and trimeric Env proteins was also conducted in Italy in volunteers at risk of HIV infection. The results indicate that vaccination is safe, immunogenic and generates strong antibody-dependent antiviral activities against both antigens [22].
Table 2. HIV-1 Tat vaccine clinical trials conducted at ISS.
| Code (ClinicalTrials.gov Identifier) |
Study | Country | Volunteers Enrolled | Reference |
|---|---|---|---|---|
| ISS P-001 (NCT00529698) | Phase I Preventive (Tat) | Italy | 20 | [20][21] |
| ISS OBS-001 (NCT01024764) | Phase I Preventive (Tat) | Italy | 20 | [20][21] |
| ISS P-002 (NCT01441193) | Phase I Preventive (Tat + Env) | Italy | 11 | Unpublished |
| ISS T-001 (NCT00505401) | Phase I Therapeutic (Tat) | Italy | 27 | [23][24] |
| ISS T-002 (NCT00751595) | Phase II Therapeutic (Tat) | Italy | 168 | [25][26] |
| ISS T-003 (NCT01513135) | Phase II Therapeutic (Tat) | South Africa | 200 | [27] |
| ISS T-002 EF-UP (NCT02118168) | Extended follow-up of ISS T-002 | Italy | 92 | [28] |
| ISS T-003 EF-UP (NCT02712489) | Extended follow-up of ISS T-003 | South Africa | 179 | Unpublished |
| Total Volunteers | 426 | |||
| Vaccinated Volunteers | 314 |
3.2. Advanced (Phase II) Clinical Development
Therapeutic vaccination represents a shorter and cost-effective route to proof-of-efficacy, as compared to preventive trials [29]. As most PLWH do not develop, or have lost, antibodies to Tat, therapeutic vaccination with Tat is an excellent setting to verify the protective role of anti-Tat immunity, especially of anti-Tat Abs, traditionally easier to investigate, as compared to cell-mediated responses, which likely play a role but are more difficult to assess [16][30][31][32][33].
Therapeutic phase II trials for cART intensification were conducted in Italy and South Africa in patients on successful cART (Table 3). The Italian phase II study (ClinicalTrials.gov Identifier: NCT00751595) was an exploratory phase II open-label therapeutic trial, randomized on the different regimens utilized [25][26]. Both primary (immunogenicity) and secondary (safety) endpoints were met. No increase in virological biomarkers was observed. In particular, a reduction in immune activation and durable increases in CD4 T cells, B cells, NK cells and CD4 and CD8 central memory T cell subsets were observed in this trial but not in subjects on effective cART, negative for anti-Tat Ab and not immunized with the Tat vaccine enrolled in a parallel observational study conducted at the same clinical centers (ISS OBS T-002) (ClinicalTrials.gov Identifier: NCT01024556) [25][26]. Of note, results from the 8-year follow-up (ClinicalTrials.gov Identifier: NCT02118168) showed anti-Tat Abs’ persistence in more than 50% of volunteers, CD4 T cells’ increases durability and the progressive decline of HIV proviral DNA, which became undetectable in the blood of 34% of all vaccinees and in 48% of volunteers who had received three times the highest dose (30 μg) of the Tat vaccine [26][28]. These results indicate that the induction of anti-Tat immune responses intensifies cART efficacy and attacks the cART-resistant virus reservoir.
Table 3. Tat vaccine phase II therapeutic clinical trials and observational follow-up studies conducted by ISS.
| Trial Name | Registration Number | Description | Immune Responses | Virological Responses | References |
|---|---|---|---|---|---|
| ISS T-002 and ISS T-002 EF-UP (extended follow-up study) | NCT00751595 and NCT02118168 | A 48-week randomized phase II, open-label, immunogenicity and safety trial in 168 anti-Tat negative HIV-1-infected cART-treated adult subjects. Volunteers intradermally received 7.5 or 30 µg of biologically active Tat, each dose given 3 or 5 times over 8 or 16 weeks. This 48-week study was followed by an extended (up to 5 years) follow-up. | Durable increase in CD4, B, and NK cell counts | HIV-1 DNA reduction 3 years after vaccination, which continued to decay in the 8 years of follow-up | [25][26][28] |
| Reduction in immune activation | |||||
| Restoration of functional CD4 and CD8 T cell subsets | |||||
| Increased T-cell responses against Env and recall antigens | |||||
| ISS T-003 and ISS T-003 EF-UP (extended follow-up study) | NCT01513135 and NCT02712489 | A 48-week randomized, double-blinded, placebo-controlled trial to evaluate immunogenicity and safety of B-clade Tat (30 μg) given intradermally, three times at 4-week intervals, in 200 HIV-infected adults on effective cART (randomized 1:1) with CD4 T cell counts ≥ 200 cells/µL. Study outcomes also included cross-clade anti-Tat antibodies, neutralization, CD4+ T-cell counts and therapy compliance. This 48-week study was followed by an extended (up to 5 years) follow-up. |
Increase in CD4 T cell counts | In patients with detectable VL at Week 48, lower geometric mean levels in vaccinees | [27] |
| Cross-clade Tat neutralizing antibodies |
A confirmatory randomized, double-blind, placebo-controlled (randomization ratio 1:1), safety and immunogenicity phase II therapeutic trial (ISS T-003, ClinicalTrials.gov Identifier: NCT01513135) was then conducted in South Africa in 200 HIV-infected (C clade) anti-Tat Ab negative adults, virologically suppressed, with CD4 T cell counts ≥200 cells/mmc [27]. The vaccine was safe and induced durable and high titers of anti-Tat Abs that cross-recognize the Tat protein from different HIV clades and cross-neutralize both clade B and C HIV viruses. Cross-recognition and cross-neutralization correlated with the increase in CD4 T cell counts, a key target for cART intensification [27]. Of note, vaccination contained the VL rebound and maintained CD4 T cell counts above the baseline levels in subjects non-compliant to therapy as compared to (non-compliant) placebo, suggesting that the Tat vaccine intensification of cART may counterbalance incomplete adherence to treatment [27]. An extended follow-up study of this trial (ISS T-003 EF-UP, ClinicalTrials.gov identifier: NCT02712489) is underway. Overall, the Tat vaccine proves for the first time that cART can be intensified by therapeutic immunization and that proviral DNA load can be progressively lowered. The data are consistent with those of Loret and colleagues, showing control of viremia upon cART interruption in volunteers vaccinated with a transactivation silent protein (Tat Oyi) [34]. In sharp contrast, no delay of virus rebound was observed upon vaccination with an immunodominant Tat peptide targeting a conserved B-cell epitope (Tat 4–12) and eliciting Abs recognizing all eight known Tat epitope variants [35], indicating that during immunization the whole Tat protein is preferable, as it triggers broad innate and adaptive immune responses, increasing the chances of the effective targeting of Tat.
References
- Deeks, S.G.; Tracy, R.; Douek, D.C. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013, 39, 633–645.
- Chen, D.; Wang, M.; Zhou, S.; Zhou, Q. HIV-1 Tat targets microtubules to induce apoptosis, a process promoted by the pro-apoptotic Bcl-2 relative Bim. EMBO J. 2002, 21, 6801–6810.
- McCloskey, T.W.; Ott, M.; Tribble, E.; Khan, S.A.; Teichberg, S.; Paul, M.O.; Pahwa, S.; Verdin, E.; Chirmule, N. Dual role of HIV Tat in regulation of apoptosis in T cells. J. Immunol. 1997, 158, 1014–1019.
- Chandrasekar, A.P.; Cummins, N.W.; Badley, A.D. The Role of the BCL-2 Family of Proteins in HIV-1 Pathogenesis and Persistence. Clin. Microbiol. Rev. 2019, 33, e00107-19.
- Krone, W.J.; Debouck, C.; Epstein, L.G.; Heutink, P.; Meloen, R.; Goudsmit, J. Natural antibodies to HIV-tat epitopes and expression of HIV-1 genes in vivo. J. Med. Virol. 1988, 26, 261–270.
- Reiss, P.; Lange, J.M.; de Ronde, A.; de Wolf, F.; Dekker, J.; Debouck, C.; Goudsmit, J. Speed of progression to AIDS and degree of antibody response to accessory gene products of HIV-1. J. Med. Virol. 1990, 30, 163–168.
- Re, M.C.; Furlini, G.; Vignoli, M.; Ramazzotti, E.; Roderigo, G.; De Rosa, V.; Zauli, G.; Lolli, S.; Capitani, S.; La Placa, M. Effect of antibody to HIV-1 Tat protein on viral replication in vitro and progression of HIV-1 disease in vivo. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1995, 10, 408–416.
- Re, M.C.; Vignoli, M.; Furlini, G.; Gibellini, D.; Colangeli, V.; Vitone, F.; La Placa, M. Antibodies against full-length Tat protein and some low-molecular-weight Tat-peptides correlate with low or undetectable viral load in HIV-1 seropositive patients. J. Clin. Virol. 2001, 21, 81–89.
- Zagury, J.F.; Sill, A.; Blattner, W.; Lachgar, A.; Le Buanec, H.; Richardson, M.; Rappaport, J.; Hendel, H.; Bizzini, B.; Gringeri, A.; et al. Antibodies to the HIV-1 Tat protein correlated with nonprogression to AIDS: A rationale for the use of Tat toxoid as an HIV-1 vaccine. J. Hum. Virol. 1998, 1, 282–292.
- Richardson, M.W.; Mirchandani, J.; Duong, J.; Grimaldo, S.; Kocieda, V.; Hendel, H.; Khalili, K.; Zagury, J.-F.; Rappaport, J. Antibodies to Tat and Vpr in the GRIV cohort: Differential association with maintenance of long-term non-progression status in HIV-1 infection. Biomed. Pharmacother. 2003, 57, 4–14.
- Rezza, G.; Fiorelli, V.; Dorrucci, M.; Ciccozzi, M.; Tripiciano, A.; Scoglio, A.; Collacchi, B.; Ruiz-Alvarez, M.; Giannetto, C.; Caputo, A.; et al. The presence of anti-Tat antibodies is predictive of long-term nonprogression to AIDS or severe immunodeficiency: Findings in a cohort of HIV-1 seroconverters. J. Infect. Dis. 2005, 191, 1321–1324.
- Bellino, S.; Tripiciano, A.; Picconi, O.; Francavilla, V.; Longo, O.; Sgadari, C.; Paniccia, G.; Arancio, A.; Angarano, G.; Ladisa, N.; et al. The presence of anti-Tat antibodies in HIV-infected individuals is associated with containment of CD4+ T-cell decay and viral load, and with delay of disease progression: Results of a 3-year cohort study. Retrovirology 2014, 11, 49.
- Cafaro, A.; Tripiciano, A.; Sgadari, C.; Bellino, S.; Picconi, O.; Longo, O.; Francavilla, V.; Buttò, S.; Titti, F.; Monini, P.; et al. Development of a novel AIDS vaccine: The HIV-1 transactivator of transcription protein vaccine. Expert Opin. Biol. Ther. 2015, 15 (Suppl. S1), S13–S29.
- Cafaro, A.; Tripiciano, A.; Picconi, O.; Sgadari, C.; Moretti, S.; Buttò, S.; Monini, P.; Ensoli, B. Anti-Tat Immunity in HIV-1 Infection: Effects of Naturally Occurring and Vaccine-Induced Antibodies Against Tat on the Course of the Disease. Vaccines 2019, 7, 99.
- Tripiciano, A.; Picconi, O.; Moretti, S.; Sgadari, C.; Cafaro, A.; Francavilla, V.; Arancio, A.; Paniccia, G.; Campagna, M.; Pavone-Cossut, M.R.; et al. Anti-Tat immunity defines CD4+ T-cell dynamics in people living with HIV on long-term cART. EBioMedicine 2021, 66, 103306.
- Cafaro, A.; Caputo, A.; Fracasso, C.; Maggiorella, M.T.; Goletti, D.; Baroncelli, S.; Pace, M.; Sernicola, L.; Koanga-Mogtomo, M.L.; Betti, M.; et al. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat. Med. 1999, 5, 643–650.
- Cafaro, A.; Bellino, S.; Titti, F.; Maggiorella, M.T.; Sernicola, L.; Wiseman, R.W.; Venzon, D.; Karl, J.A.; O’Connor, D.; Monini, P.; et al. Impact of viral dose and major histocompatibility complex class IB haplotype on viral outcome in mauritian cynomolgus monkeys vaccinated with Tat upon challenge with simian/human immunodeficiency virus SHIV89.6P. J. Virol. 2010, 84, 8953–8958.
- Maggiorella, M.T.; Baroncelli, S.; Michelini, Z.; Fanales-Belasio, E.; Moretti, S.; Sernicola, L.; Cara, A.; Negri, D.R.; Buttò, S.; Fiorelli, V.; et al. Long-term protection against SHIV89.6P replication in HIV-1 Tat vaccinated cynomolgus monkeys. Vaccine 2004, 22, 3258–3269.
- Borsetti, A.; Baroncelli, S.; Maggiorella, M.T.; Moretti, S.; Fanales-Belasio, E.; Sernicola, L.; Tripiciano, A.; Macchia, I.; Michelini, Z.; Belli, R.; et al. Containment of infection in tat vaccinated monkeys after rechallenge with a higher dose of SHIV89.6P(cy243). Viral Immunol. 2009, 22, 117–124.
- Ensoli, B.; Fiorelli, V.; Ensoli, F.; Lazzarin, A.; Visintini, R.; Narciso, P.; Di Carlo, A.; Tripiciano, A.; Longo, O.; Bellino, S.; et al. The preventive phase I trial with the HIV-1 Tat-based vaccine. Vaccine 2009, 28, 371–378.
- Bellino, S.; Francavilla, V.; Longo, O.; Tripiciano, A.; Paniccia, G.; Arancio, A.; Fiorelli, V.; Scoglio, A.; Collacchi, B.; Campagna, M.; et al. Parallel conduction of the phase I preventive and therapeutic trials based on the Tat vaccine candidate. Rev. Recent Clin. Trials 2009, 4, 195–204.
- Ensoli, B. National HIV/AIDS Research Center. Istituto Superiore di Sanità: Rome, Italy, 2024; manuscript in preparation.
- Ensoli, B.; Fiorelli, V.; Ensoli, F.; Lazzarin, A.; Visintini, R.; Narciso, P.; Di Carlo, A.; Monini, P.; Magnani, M.; Garaci, E. The therapeutic phase I trial of the recombinant native HIV-1 Tat protein. AIDS 2008, 22, 2207–2209.
- Longo, O.; Tripiciano, A.; Fiorelli, V.; Bellino, S.; Scoglio, A.; Collacchi, B.; Alvarez, M.J.R.; Francavilla, V.; Arancio, A.; Paniccia, G.; et al. Phase I therapeutic trial of the HIV-1 Tat protein and long term follow-up. Vaccine 2009, 27, 3306–3312.
- Ensoli, B.; Bellino, S.; Tripiciano, A.; Longo, O.; Francavilla, V.; Marcotullio, S.; Cafaro, A.; Picconi, O.; Paniccia, G.; Scoglio, A.; et al. Therapeutic immunization with HIV-1 Tat reduces immune activation and loss of regulatory T-cells and improves immune function in subjects on HAART. PLoS ONE 2010, 5, e13540.
- Ensoli, F.; Cafaro, A.; Casabianca, A.; Tripiciano, A.; Bellino, S.; Longo, O.; Francavilla, V.; Picconi, O.; Sgadari, C.; Moretti, S.; et al. HIV-1 Tat immunization restores immune homeostasis and attacks the HAART-resistant blood HIV DNA: Results of a randomized phase II exploratory clinical trial. Retrovirology 2015, 12, 33.
- Ensoli, B.; SMU-MeCRU Study Group; Nchabeleng, M.; Ensoli, F.; Tripiciano, A.; Bellino, S.; Picconi, O.; Sgadari, C.; Longo, O.; Tavoschi, L.; et al. HIV-Tat immunization induces cross-clade neutralizing antibodies and CD4+ T cell increases in antiretroviral-treated South African volunteers: A randomized phase II clinical trial. Retrovirology 2016, 13, 34.
- Sgadari, C.; Monini, P.; Tripiciano, A.; Picconi, O.; Casabianca, A.; Orlandi, C.; Moretti, S.; Francavilla, V.; Arancio, A.; Paniccia, G.; et al. Continued Decay of HIV Proviral DNA Upon Vaccination With HIV-1 Tat of Subjects on Long-Term ART: An 8-Year Follow-Up Study. Front. Immunol. 2019, 10, 233.
- Ensoli, B.; Cafaro, A.; Monini, P.; Emarcotullio, S.; Ensoli, F. Challenges in HIV Vaccine Research for Treatment and Prevention. Front. Immunol. 2014, 5, 417.
- van Baalen, C.A.; Huisman, R.C.; Klein, M.R.; Gruters, R.A.; Miedema, F.; Geretti, A.M.; Osterhaus, A.D.; Pontesilli, O.; de Wolf, F. Human immunodeficiency virus type 1 Rev- and Tat-specific cytotoxic T lymphocyte frequencies inversely correlate with rapid progression to AIDS. J. Gen. Virol. 1997, 78, 1913–1918.
- Addo, M.M.; Altfeld, M.; Rosenberg, E.S.; Eldridge, R.L.; Philips, M.N.; Habeeb, K.; Khatri, A.; Brander, C.; Robbins, G.K.; Mazzara, G.P.; et al. The HIV-1 regulatory proteins Tat and Rev are frequently targeted by cytotoxic T lymphocytes derived from HIV-1-infected individuals. Proc. Natl. Acad. Sci. USA 2001, 98, 1781–1786.
- Cao, J.; McNevin, J.; Malhotra, U.; McElrath, M.J. Evolution of CD8+ T cell immunity and viral escape following acute HIV-1 infection. J. Immunol. 2003, 171, 3837–3846.
- Jones, N.A.; Wei, X.; Flower, D.R.; Wong, M.; Michor, F.; Saag, M.S.; Hahn, B.H.; Nowak, M.A.; Shaw, G.M.; Borrow, P. Determinants of human immunodeficiency virus type 1 escape from the primary CD8+ cytotoxic T lymphocyte response. J. Exp. Med. 2004, 200, 1243–1256.
- Loret, E.P.; Darque, A.; Jouve, E.; Loret, E.A.; Nicolino-Brunet, C.; Morange, S.; Castanier, E.; Casanova, J.; Caloustian, C.; Bornet, C.; et al. Intradermal injection of a Tat Oyi-based therapeutic HIV vaccine reduces of 1.5 log copies/mL the HIV RNA rebound median and no HIV DNA rebound following cART interruption in a phase I/II randomized controlled clinical trial. Retrovirology 2016, 13, 21, Erratum in: Retrovirology 2016, 13, 35.
- Goldstein, G.; Damiano, E.; Donikyan, M.; Pasha, M.; Beckwith, E.; Chicca, J. HIV-1 Tat B-cell epitope vaccination was ineffectual in preventing viral rebound after ART cessation: HIV rebound with current ART appears to be due to infection with new endogenous founder virus and not to resurgence of pre-existing Tat-dependent viremia. Hum. Vaccines Immunother. 2012, 8, 1425–1430.
This entry is offline, you can click here to edit this entry!
