Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Biomedical
细胞外囊泡分布的外泌体携带与细胞高度一致的各种信息,成为肿瘤筛查的新型生物标志物。然而,虽然传统的表征技术可以量化外泌体的大小和形态,但在功能追踪、单位点蛋白质定量和微观结构信息等相关领域受到限制。
- exosome
- tumor diagnosis
- optical analysis technology
- super-resolution microscope
1. 引言
外泌体是循环肿瘤细胞和循环肿瘤DNA后的新型肿瘤生物标志物[1,2,3],可用于肿瘤诊断[4]。外泌体的生物信号分子通过内吞作用进行交换,从而调节肿瘤细胞的生长、转移、耐药和免疫逃逸等活动[5,6]。因此,外泌体在肿瘤的早期诊断、治疗进展监测和预后方面具有重要价值,如图1所示[4,7,8,9]。
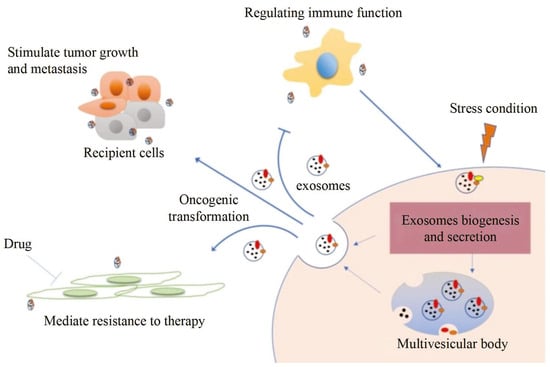
图 1.外泌体信息传递过程及应用[4]。
外泌体是由粒径为30-150nm的细胞分泌的膜状细胞外囊泡(EV)[10]。研究发现,外泌体的功能取决于它们来源的细胞类型,同时它保持与供体细胞相同的遗传物质[11]。在外泌体活性期间,不同的分析成为肿瘤特异性研究靶点之一。例如,来源于肿瘤细胞的外泌体携带多种类型的蛋白质,如表面蛋白、内含物、酶等。其中,CD9、CD63、CD81等表面蛋白和HSP70、Alix等包涵因子是分离鉴定外泌体的代表性蛋白[12,13,14]。蛋白质的差异可以反映肿瘤细胞与基底细胞之间以及肿瘤细胞与肿瘤细胞之间的信息交换,从而调节免疫应答、迁移、分化和其他基本细胞功能[15,16,17,18]。例如,研究发现,来源于不同细胞的外泌体在大小、形态和组成上是不同的[19]。来源于肿瘤细胞的外泌体较大,含有较多的脂质和外膜蛋白,可促进肿瘤细胞生长、侵袭和转移[20]。癌症外泌体的表面蛋白在不同阶段往往不同,这表明这些蛋白质与癌症的发生过程密切相关。同样,不同来源的癌症外泌体的表面蛋白也不同,可用于癌症的早期诊断[21,22]。研究证明,ADAM10、金属蛋白酶、CD9、膜联蛋白-1和HSP70在从乳腺癌患者胸腔积液或血清中分离的外泌体中富集[23]。然而,来自免疫细胞的外泌体较小,包括多种免疫分子,如细胞因子、抗原、抗体等,可以调节免疫反应和抗肿瘤作用[24]。
2. 常规表征技术
2.1. 可调电阻脉冲检测
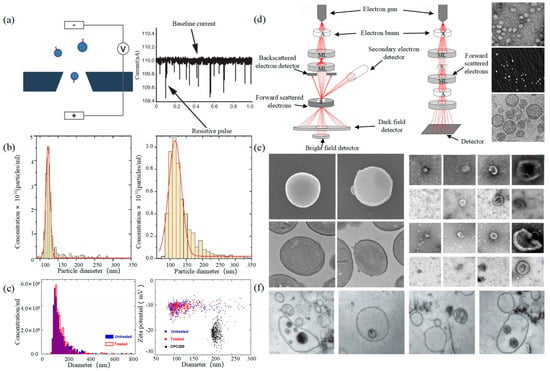
可调电阻脉冲检测 (TRPS) 基于库尔特原理。将悬浮液混合在电解液中,电解液可以通过具有特定孔径的纳米孔芯片。两个电极内外之间的电阻在通过纳米孔的那一刻瞬时发生变化,其结果是脉冲信号,如图2所示。信号的强度和频率与外泌体的大小和数量有关。通过脉冲信号计数外泌体的表达。

图2.TRPS和EM (a) TRPS的原理[43]。(b)TRPS揭示了源自TIME细胞系(左)和HUVEC细胞系(右)的EV的大小分布[45]。(c)INTERCEPT处理前后EV样品的颗粒浓度和粒径测量(左),未处理和INTERCEPT处理的EV样品和羧基化聚苯乙烯标准颗粒(右)的尺寸和zeta电位测量(右)[46]。(d) SEM(左)和TEM(中)[47]的原理以及TEM [48]、SEM [49]、cryo-TEM [50](右)的EVs扫描原理。(e)无EV和有EV的金黄色葡萄球菌的EM图像(左)[51]。不同来源的单个杯形EV的EM图像示例[19]。(f)培养的HMC-1细胞和周围生长培养基中的细胞外囊泡中含有的多囊泡体(MVB)[52]。
2.2. 电子显微镜
电子显微镜(EM)是测量单个EV尺寸和形态的最直接方法[42]。它分为扫描电子显微镜(SEM)、透射电子显微镜(TEM)和冷冻透射电子显微镜(cryo-TEM)。值得注意的是,EM可以表征单个EV的颗粒形态和大小[53,54]。
通过聚焦电子束对EV的外膜进行成像,其结果是具有不同发射量的二次电子。原理如图 2 左侧所示。Min Kyo Jung等观察了阴性染色技术染色的EVs的详细结构和特异性蛋白[55]。Boz等通过SEM观察了hbEV的机制和形态[56]。Sokolova等人获得了来自不同细胞(包括HEK110T、ECFC和MSC)的外泌体的形态学特征[57]。透射电镜的原理是电子束与EVs的原子碰撞,并转换散射角以获得结构的变化,如图2中间所示。
2.3. 动态光散射
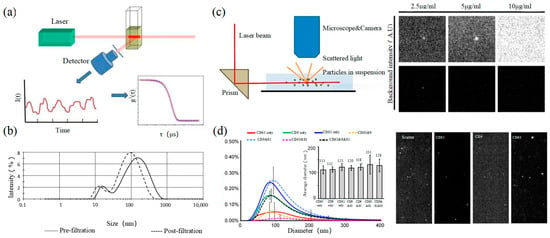
动态光散射(DLS)是一种测量亚微米颗粒尺寸和分布的光学分析方法,其基本原理如图3所示。粒子的布朗运动引起光散射信号的变化,该信号由数字自相关器监测,以计算粒子的扩散速度和粒子分布[62]。小颗粒的运动速率更高,其强度波动更大。结果,相关曲线迅速而明显地下降。DLS的灵敏度和FOV均优于上述论文,可实现大尺寸外泌体纳米颗粒的基本表征。对于较小的颗粒,自聚合行为会干扰光强信号。因此,无法实现高浓度样品的精密分析和检测。2009年,Lawrie等人使用DLS表征了源自红细胞的EV的大小分布[63]。DLS同时分析样品中的所有颗粒,因此无法提供有关某一类颗粒的数量或浓度的信息[64]。

图3.DLS和NTA (a)DLS的原理[67]。(b)DLS表征新鲜冷冻血浆中微粒的尺寸分布[68]。(c) NTA(左)的原理(左)[69]和细胞外囊泡荧光标记的EVs和NTA的荧光检测能力[70]。(d) 三种CD标记物(CD9、CD63和CD81)在NTA(左)和NTA(右)的颗粒荧光图像中的尺寸分布[70]。
2.4. 纳米粒子跟踪分析
Nanoparticle tracking analysis (NTA) is an optical method to assess nanoparticles [71]. When the laser irradiates a single nanoparticle, light intensity scattered from the sample is captured by a high-speed camera as shown in the left of Figure 3. Compared with DLS, NTA can locate and track a single nanoparticle, so this technology has advantages in analyzing the particle size of complex samples. It is worth mentioning that NTA has the ability of fluorescence analysis. Different fluorescent particles can be measured by the relevant parameters, which can identify the sizes of various exosomes at the same time. In 2011, Dragovic et al. applied quantum dot-labeled fluorescent cell tracking peptide with NTA to confirm the origin of common vesicles in plasma [72].
2.5. Flow Cytometry
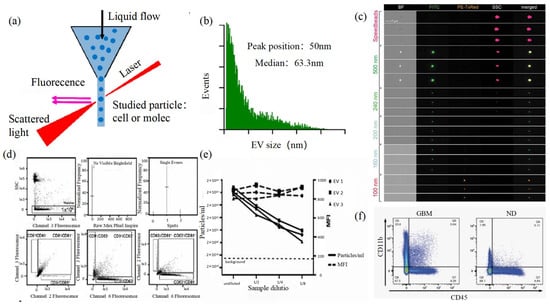
Size and morphology are the basic parameters for the characterization of exosomes. The characterization of functional parameters such as surface protein quantitative expression and signal transduction mode is of paramount importance. Flow Cytometry (FCM) realizes the rapid multi-parameter quantitative analysis of cells or submicron particles based on light scattering changes, and its basic principle is shown in Figure 4. Scatters of light from particles suspended in a sheath stream reflect the size and density of the cells or particles, and they were acquired by a detector array. At the same time, the specific gene expression, protein expression, enzyme activity, ion concentration, and other biomolecular substances labeled by fluorescent dyes were specifically measured by different channels.
The sensitivity of traditional flow cytometry is limited to 300 to 500 nm [76], so it is obviously difficult to measure exosomes. Yan Xiaomei’s team developed nFCM by combining Rayleigh scattering with sheath flow single-molecule fluorescence detection technology, which enables the high-throughput analysis of exosomes with a size of 40 nm, as shown in Figure 4 [77,78]. Compared with traditional flow cytometry, the scattered light detection sensitivity of nFCM is improved by four to six orders.

Figure 4. FCM (a) The principle of FCM [69]. (b) Histogram of particle size for an EV sample of HCT-15 cells [78]. (c) Heterogeneous fluorescent beads were analyzed by FCM [80]. (d) FCM detects CD9, CD63, and CD81 positive EVs [80]. (e) FCM analyzes the EV concentration and mean fluorescence intensity (MFI) of the anti-CD9-PE-stained EVs of the three individual EV preparations [80]. (f) Analysis of plasma EVs expressing CD45 and CD11b in GBM and normal donors [81].
3. Super-Resolution Imaging Technology
The development of modern biology has promoted the rapid progress of microscopic imaging technology. Due to the limitation of optical diffraction, the minimum resolution of the traditional optical microscope is about half of the wavelength of incident light. Therefore, scientists have been constantly trying to break through it. The resolution of super-resolution imaging technology is below 200 nm, which obtains the same level of resolution as EM. Super-resolution microscopy can achieve real-time super-resolution imaging for organelle structure, interaction, protein function, etc. It provides a new analytical method for cell biology and breaks through the biomedical research status from the nano-scale.
At present, super-resolution imaging technology is divided into two categories. One is a single molecule localization imaging technique (SMLM) based on the random switching of the excited light of the fluorophores between on and off states, which includes stochastic optical reconstruction microscopy (STORM) and photoactivated localization microscopy (PALM). The other is the super-resolution imaging technology achieved based on light field regulation such as stimulated emission depletion (STED) and structured illumination microscopy (SIM).
3.1. Single Molecule Localization Imaging Technology
The basic principle of SMLM is based on the flicker of a single fluorescent molecule to locate a single molecule and then reconstruct super-resolution images. Compared with other technologies, SMLM has the advantages of low phototoxicity and low cell damage. It is more suitable for living cells, thus becoming a new super-resolution analysis method for exosomes in vivo observation. SMLM opens a new observation perspective for exosome-related studies.
3.1.1. Stochastic Optical Reconstruction Microscopy and Photoactivated Localization Microscopy Technology
PALM and STORM technologies are classical technologies in SMLM. In 2006, Eric Betzig et al. proposed the PALM technology [82], and Xiaowei Zhuang et al. proposed the STORM technology [83] at the same time. Both of them are based on single-molecule localization technology to achieve the super-resolution imaging of subcellular structure molecules. One of the key elements is the switched fluorophores. For example, PALM uses photoactivated green fluorescent protein (PA-GFP) to label the protein and irradiate the cell surface with different lasers so as to cause the fluorescence molecule cycle to complete the excitation localization process.
One of the key points is the spatial and temporal resolution for SMLM. That requires more than 10,000 frames of images during the process of reconstruction, which needs much more time. The rapid development of EMCCD cameras has greatly improved imaging speed. In 2011, Zhuang Xiaowei’s group pictured extracellular vesicles with a high-speed EMCCD. The temporal resolution was improved to 0.5 s, which means that STORM has the potential to monitor live cell imaging in real-time [85].
In 2011, Zhuang’s team used the stage-specific neurite-associated protein (SNAP) label to label the Alexa Flour 467 optical switching probe to clathrin in living BS-C-1 cells. STORM technology was successfully used to obtain a 30 nm horizontal resolution and a 50 nm vertical resolution [85]. In 2012, Shim et al. determined the STORM membrane probe for live cell imaging through a large number of experiments and performed the super-resolution imaging of organelle membranes in live cells, reaching a spatial resolution of 20~60 nm [92]. In 2018, Zong Shenfei et al. discovered that silicon quantum dots (Si QD) have fluorescent scintillation behavior and applied them as SMLM imaging nanoprobes to stain CD63 of breast cancer cell (SKBR3)-derived EVs using CD63 aptamers fused with Si QD, achieving an imaging accuracy of about 30 nm. They demonstrated that Si QD can be used for the SMLM imaging of small objects such as exosomes. Moreover, Si QD has the characteristics of high biocompatibility and low cytotoxicity, which makes it a better choice of fluorophores for SMLM live cell imaging [93].
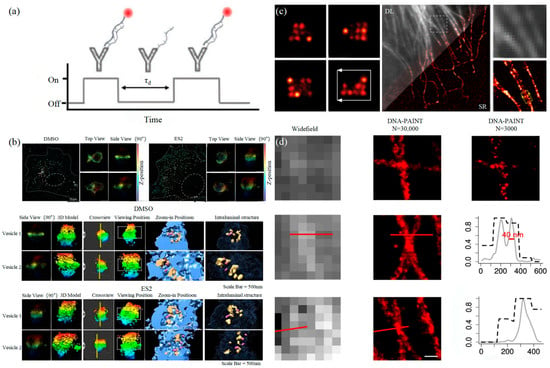
3.1.2. DNA-PAINT Technology
Similar to the STORM/PALM technology, DNA-PAINT also achieves super-resolution imaging by controlling the flicker of individual fluorophores. In 2014, Ralf Jungmann et al. proposed the DNA-PAINT technology, which uses reversible binding between complementary DNA sequences to produce an effect similar to the “flicker” of fluorescent molecules [101]. In double-strand DNA, one strand is connected to the fluorophores, called the imager strand, and the other is connected to the target molecule, called the docking strand. Due to the highly specific binding of the double-strand DNA, the imaging strand and the docking strand are bound spontaneously, producing single-molecule fluorescence in the focal plane [102] as shown in Figure 6. In addition, multi-channel fluorescence imaging can be realized by different proteins labeled with various docking strands.

Figure 6. DNA-PAINT (a) The principle of DNA-PAINT [103]. (b) 3D DNA-PAINT images of COS-7 cells treated with DMSO or ES2 [104]. (c) Representative super-resolution images of 20 nm grid structures (left) and Diffraction-limited (DL) alongside the super-resolution (SR) of the microtubule network in a HeLa cell [105]. (d) Comparison of the widefield images and DNA-PAINT images [106].
The DNA-PAINT technology focused on reducing the resolution to the molecular level early. Then, it focused on the limitation of long acquisition times [107]. Long collection time is a basic limitation of SMLM technology, which is due to the need to collect enough photons to determine the central position of the fluorophores [108]. The common idea is to increase the binding frequency at a high concentration of the docking strand, but the concentration of the imaging strand influences the SNR of technology [109].
Proteins on exosomes can be quantitatively analyzed with high precision by DNA-PAINT. The different contents of proteins carried by exosomes such as CD9, CD63, CD81, HER2, EpCAM, and EGFR could achieve the classification of the course of tumorigenesis [111]. Chen et al. combined DNA-PAINT with machine learning to identify tumor cell types in two steps [103]. The first step is to distinguish between healthy cells and tumor cells and then to perform cross-typing on different types of tumor cells, among which the comparison results of breast cancer and pancreatic cancer show that the system can accurately and quickly distinguish the two types of cancer, which makes it possible for exosomes to achieve an early diagnosis of cancer through optical detection.
3.2. Stimulated Emission Depletion Technology
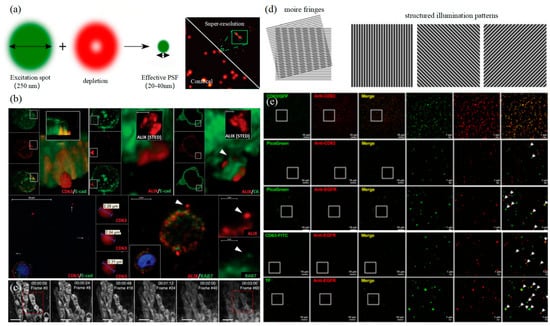
In 1994, Hell et al. proposed stimulated emission depletion technology (STED) for the first time in theory. The principle of STED is that two laser beams are used for microscopic imaging, with one as excitation light for photon excitation and the other as loss light, which is a ring laser with zero central intensity [113]. The loss light should be coaxial with the excitation light, and the wavelength should match the emission wavelength of the fluorescence molecule as shown in the left of Figure 7. STED is a super-resolution imaging technology developed based on laser scanning confocal microscopy. Fast, direct imaging characteristics and a nanoscale observation scale are ideal for living cell research. However, the resolution of STED is affected by the ratio of lost optical power to probe saturation excitation power. The higher the relative power, the higher the imaging resolution, but it will also cause the rapid photobleaching of fluorophores, causing serious photodamage to cells. Therefore, the application of STED to live cell super-resolution requires the development of fluorescent probes.

图7.STED和SIM (a)STED的原理(左)[123]以及使用STED标记UCNPs的单EV表征(右)[121]。(b) HT29结直肠癌细胞中细胞外sEVC的可视化[116]。(c)线粒体的延时STED成像[117]。(d) SIM卡原理(左)[123]。(e)通过基于遗传标签(CD63-GFP)和CD63免疫荧光成像之间的完全共定位来验证SIM分辨率[124]。
3.3. 结构照化显微镜技术
结构照度显微镜技术(SIM)是Gustafsson于2000年提出的一种基于频域调制的超分辨率成像技术[125]。莫尔条纹用于将系统截止频率之外的高频信息传输到低频部分,以实现信号检测并提高成像分辨率。激发光可以通过炉排产生正弦条纹图案来照射样品,如图 7 左侧所示。SIM卡通常只需要拍摄九帧图像,时间分辨率要高得多。此外,激发光强度也相对较低,适用于活细胞。然而,由于SIM受到成像原理的限制,横向分辨率只能达到150纳米左右,这意味着SIM虽然与其他超分辨率成像技术相比具有时间分辨率的优势,但受到空间分辨率的限制,无法满足外泌体的检测要求。
4. 总结
随着科学技术的发展,光学技术已成为探索外泌体生物发生和作用机制的主要手段,尤其是超分辨率成像技术。它不仅突破了衍射限制,而且实现了三色和多色成像。它极大地结合了传统表征技术的优势,并促进了基于外泌体的各种生物医学研究,例如肿瘤的早期诊断和分型。SIM 和 STED 可能会由于光漂白、光毒性和缺乏分辨率而限制外泌体的进一步研究和应用。无论是算法优化还是荧光探针的开发,都促进了两者的优化,尤其是非线性SIM技术的发展,促进了其在外泌体中的应用。相比之下,SMLM对外泌体的研究具有广泛的应用前景。例如,STORM技术具有超分辨率和快速实时分析的优势,可以清晰直观地分析外泌体的三维形态和细胞内外的功能信息。基础研究的结果可以带来更多的医学应用。陈晨的团队为我们树立了良好的榜样。目前,研究人员也在积极开发各种方法来改进超分辨率成像技术,以促进外泌体的精准医疗。
This entry is adapted from the peer-reviewed paper 10.3390/photonics11020101
This entry is offline, you can click here to edit this entry!
