Circa il 15% degli antibiotici viene prescritto per il trattamento delle infezioni del tratto urinario (UTI), che sono alcune delle infezioni batteriche più comuni. Le infezioni delle vie urinarie colpiscono oltre 400 milioni di persone ogni anno e provocano 150 milioni di decessi in tutto il mondo [
1 ,
2 ]. Si stima che circa il 50% delle donne e il 5% degli uomini sperimenteranno una IVU nel corso della loro vita [
3 ]. L’incidenza delle infezioni del tratto urinario aumenta con l’età, colpendo tra il 30% e il 50% delle donne di età superiore ai 60 anni [
4 ]. La patogenesi delle infezioni delle vie urinarie è il risultato di numerose interazioni complesse tra un uropatogeno e l'ospite [
5 ,
6 ]. Tuttavia, è ampiamente riconosciuto che l’uso ambulatoriale di antibiotici è spesso eccessivo e non necessario [
7 ]. Sebbene gli antibiotici siano stati usati per trattare le infezioni comuni per molti anni, la resistenza agli antibiotici ha reso inefficaci diversi antibiotici comunemente usati per le infezioni del tratto urinario, portando a malattie più gravi, ricoveri ospedalieri e decessi, nonché ad un aumento dei costi sanitari [
6 ,
8 ] . La resistenza agli antibiotici è una delle principali preoccupazioni e le infezioni delle vie urinarie resistenti agli antibiotici rappresentano un aspetto critico [
9 ]. La resistenza agli antibiotici è stata identificata dall’Organizzazione Mondiale della Sanità come una delle dieci principali minacce globali alla salute pubblica nel 2021 [
10 ]. Un'infezione del tratto urinario che si diffonde nel flusso sanguigno e causa sepsi può essere fatale e le infezioni resistenti ai farmaci possono renderlo più probabile [
6 ,
11 ]. Nel 2019, le infezioni resistenti ai farmaci hanno causato direttamente 1,27 milioni dei circa 4,95 milioni di decessi attribuiti alla resistenza agli antibiotici. Ciò ha superato il numero di decessi causati dall’HIV/AIDS [
12 ,
13 ]. La resistenza agli antibiotici è un fenomeno naturale, ma la sua accelerazione dovuta all’abuso di antibiotici nell’uomo e negli animali costituisce un problema serio [
14 ]. Ciò è particolarmente preoccupante per le infezioni del tratto urinario, che sono tra le malattie infettive più diffuse [
15 ]. I biofilm sono ampiamente riconosciuti come uno dei principali fattori che contribuiscono agli alti tassi di recidiva e resistenza agli antibiotici comunemente associati alle infezioni del tratto urinario [
16 ,
17 ]. I biofilm possono essere formati da diversi tipi di batteri, comprese le specie Gram-positive e Gram-negative, ed è noto che svolgono un ruolo importante in diversi processi patologici [
18 ,
19 ].
2. Classificazione e patogenesi delle IVU
2.1. Tipi di infezioni del tratto urinario
There are several classification systems for UTIs [
20]. However, the most widely used systems are those developed by the CDC, which distinguish between uncomplicated and complicated UTIs on the basis of the presence of risk factors such as anatomical or functional abnormalities in the urinary tract [
5,
6,
21]. UTIs are also classified according to the location of the infection and the clinical presentation of the UTI [
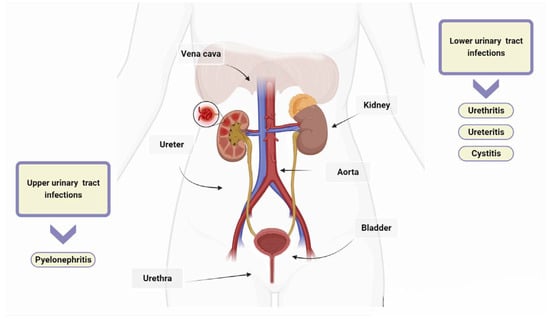
6]. The urinary tract consists of the kidneys, ureters, bladder, and urethra. The kidneys filter waste products from the blood and produce urine, which then passes through the ureters to the bladder. UTIs are typically categorized as upper or lower depending on their location within the urinary tract. Urethritis is an inflammation of the urethra, and ureteritis is an inflammation of the ureter [
22]. Lower UTIs, including cystitis, which refers to a bladder infection, and upper UTIs, such as pyelonephritis involving the kidney, are classified based on the affected area [
23]. In healthy, pre-menopausal, non-pregnant women with no history of an abnormal urinary tract, acute cystitis and pyelonephritis are the classifications for uncomplicated UTIs, with women being more commonly affected than men [
24]. Complicated UTIs involve functional or metabolic abnormalities, such as obstruction, urolithiasis, pregnancy, diabetes, neurogenic bladder, renal, or other immunocompromising conditions [
25]. Recurrence, catheter association, and urosepsis are also included in the classification of UTIs [
26]. UTIs are classified as recurrent if two or more episodes occur within six months or three or more episodes occur within 12 months [
27,
28]. Catheter-associated urinary tract infections (CAUTIs) account for around 75% of all hospital-acquired UTIs [
29]. The risk of contracting these infections increases with prolonged use of a urinary catheter, which is a tube inserted into the bladder through the urethra to drain urine. Urosepsis is a systemic inflammatory response to UTIs such as cystitis, bladder infection, and pyelonephritis, and it can lead to multiple organ dysfunction, failure, and even death [
30].
2.2. Clinical Syndromes
Diagnosing UTIs can be challenging, especially in patients with non-specific symptoms [
31]. However, it is crucial to differentiate between UTIs and asymptomatic bacteriuria to determine the necessity of antibiotic treatment [
6]. Asymptomatic bacteriuria is the presence of one or more bacterial species in urine, as indicated by a positive urine culture, in a patient who does not exhibit any signs or symptoms of a UTI (see below) [
32]. In contrast, UTIs are infections that can affect the urethra, bladder, ureters, and/or kidneys. The symptoms experienced depend on which part of the urinary tract is affected [
4,
32].
Table 1 shows the signs and symptoms of UTIs depending on the location of the bacterial infection.
Table 1. Signs and symptoms of UTIs in different parts of the urinary system.
UTIs are more common in women and typically affect the bladder and urethra [
18]. Uropathogens commonly infect the urinary tract through an ascending route that starts in the genital area, passes through the urethra to the bladder, and then ascends the ureters to reach the kidneys (
Figure 1). Cystitis is a prevalent UTI among women, especially pregnant women, due to pregnancy’s interference with bladder emptying [
33]. Women are more susceptible to cystitis due to the shorter length of their urethra and its proximity to the anus. Recurrent episodes of cystitis are common among women, especially during their reproductive years, as bacteria can travel from the urethra to the bladder during sexual intercourse [
34]. Bladder infections in women can also be caused by the use of a diaphragm for contraception or the decrease in estrogen production after menopause [
35]. The spermicide used in conjunction with a diaphragm suppresses the natural vaginal flora, allowing the bacteria responsible for cystitis to thrive [
21]. Bladder prolapse can occur as a result of decreased estrogen production during menopause, which can make it difficult to empty the bladder and increase the risk of bladder infections [
36]. In men, cystitis and urethritis are frequently caused by bacterial prostatitis resulting from ascending urethral infection or intraprostatic reflux [
37]. If antibiotics fail to eliminate all bacteria, such as when they do not penetrate the prostate adequately or when treatment is discontinued prematurely, prostatitis may recur and become chronic [
38]. Bacteria that persist in the prostate gland have a tendency to re-infect the bladder. Cystitis can also result from a urethral stone obstructing urine flow, preventing the removal of residual bacteria that can rapidly multiply in the bladder, or from a catheter that introduces bacteria into the bladder [
4]. Urethritis is an infection of the urethra, which is the tube that carries urine from the bladder to the outside of the body. Symptoms of urethritis typically include pain during urination, a strong urge to urinate, and sometimes discharge (which is more common in men than women) [
39]. The most common causes of urethritis are sexually transmitted microorganisms, such as
Neisseria,
Chlamydia,
Herpes simplex virus, and
Trichomonas [
40]. Pyelonephritis is a type of urinary tract infection (UTI) that typically originates in another part of the urinary tract, such as the urethra or bladder, and then spreads to one or both kidneys [
41]. In rare cases (about 5%), infections can also spread to the kidneys from other parts of the body through the bloodstream [
4]. Pyelonephritis is more common in women than in men and may be milder and more difficult to recognize in children and the elderly [
41]. Risk factors of pyelonephritis include kidney stones, catheterization, urinary anatomical abnormalities, reflux, diabetes mellitus, enlarged prostate, and pregnancy [
6].
Figure 1. Urinary tract infections (UTIs). UTIs are classified as upper or lower based on their anatomical location. Lower UTIs are further categorised by the specific anatomical part affected, such as urethritis for the urethra, ureteritis for the ureter, and cystitis for the bladder. If microorganisms evade the host’s defences and are not promptly eradicated, they can travel from the lower urinary tract to the kidneys and, if left untreated, can lead to pyelonephritis.
2.3. Urinary Tract Infections Caused by Bacteria
UTIs are caused by both Gram-negative and Gram-positive bacteria [
42]. Uropathogenic
Escherichia coli is the most common pathogen found in both uncomplicated and complicated UTIs. This bacterium is responsible for at least 80% and 65% of community- and hospital-acquired UTIs, respectively [
6]. For several decades, it was believed that the bladder and urethra of healthy individuals were sterile or contained too few bacteria to cause infections [
43]. However, recent studies have shown evidence of numerous microorganisms in the bladder of adult humans without clinical infections. The relevance and contribution of these microorganisms to health and disease are still under investigation [
43,
44]. Common pathogens causing UTIs include
Enterococcus faecalis,
Proteus mirabilis,
Klebsiella pneumoniae,
Staphylococcus saprophyticus,
Group B Streptococcus,
Pseudomonas aeruginosa, and
S. aureus [
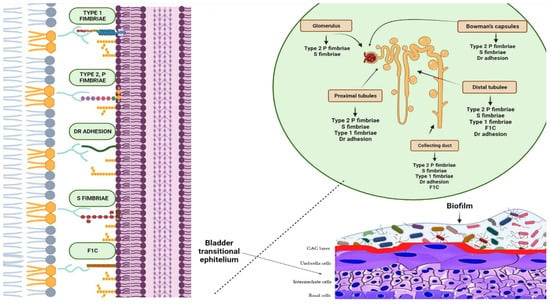
6]. Uropathogens express fimbrial adhesins that bind to glycolipids and glycoproteins on the mucosal–epithelial surface of the host (
Figure 2) [
45]. Adhesins are used by uropathogens to create biofilms on both biotic and abiotic surfaces, enabling them to colonise the mucosa of the urinary tract and indwelling devices such as urinary catheters [
46]. Furthermore, some pathogens secrete substances, such as hemolysin, capsule, proteases, and phospholipases, which aid bacterial invasion by disrupting epithelial integrity [
45,
47,
48,
49,
50]. Additionally, certain uropathogens, such as UPEC, can invade the host’s epithelial cells and replicate within them, creating a reservoir for recurrent infections [
51]. This enables the bacteria to colonise and cause UTIs. Bacterial virulence factors, such as fimbriae, adhesins, P and type 1 pili, and others that facilitate bacterial invasion, have been described in detail in previous comprehensive reviews (
Figure 2) [
4,
6,
45,
52].
Figure 2. Biofilms are bacterial formations that occur on urothelial surfaces. The spread and colonisation of uropathogens is facilitated by several virulence factors, including adhesion proteins. The figure displays bacterial adhesins that bind to specific host cell membrane structures, such as glycosphingolipids, sialic acid-containing receptors, and glycolipid and mannose receptors, which are expressed in different tracts of the uroepithelium. Type 1 fimbriae facilitate bacterial adhesion at the proximal and distal tubules, as well as at the level of the collecting duct. Bacteria expressing type 2 and S fimbriae can adhere to various parts of the urinary tract, including the glomeruli, Bowman’s capsule, collector duct, and proximal and distal tubules. Adhesion is also possible at the level of the bladder, collecting ducts, and distal and proximal tubules through Dr adhesins. Furthermore, bacteria that are able to colonise the bladder, distal tubule, and collector duct express the adhesion protein F1C, which can result in the formation of biofilms.
3. The Role of Biofilm in the Persistence and Recurrence of UTIs
Biofilm cells have distinct characteristics compared to those of planktonic cells, including increased antibiotic resistance and evasion of the innate and adaptive immune response [
46]. These characteristics arise from the unique structure of biofilms and the activation of various processes. The high viscosity of the biofilm obstructs the host’s immune defence system and antimicrobial treatments, contributing to its resistance and persistence [
73]. Furthermore, the release of extracellular toxins results in significant tissue damage, leading to an increase in nutrient availability and further consolidation of the biofilm [
74]. Asynchronous microbial growth within the biofilm gives rise to variant bacterial phenotypes, such as persister cells, which are inherently resistant to antibiotics [
60]. Biofilm-forming bacteria exhibit phenotypic variations, which increase the rate of horizontal gene transfer (HGT). HGT can occur through several mechanisms, including conjugation, transformation, and transduction [
75]. It is important to note that while these mechanisms are well known, they are not the only ones. Recently, a fourth mechanism has been identified, which involves membrane vesicles that transfer antibiotic resistance genes between members of the biofilm community [
76]. CAUTIs caused by bacterial biofilms are a common type of hospital-acquired infection [
69]. Urinary catheters are drainage tubes made of silicone or latex that are inserted into the bladder to collect urine. They are commonly used during or after surgery to reduce overflow incontinence or to relieve urinary retention [
69].
E. coli,
K. pneumoniae, and
P. mirabilis are the most common causes of CAUTIs [
77]. Bacteria attach to the surface of the catheter and produce urease, which hydrolyses urea into ammonium ions [
78]. This process raises the pH of the urine, resulting in the formation of crystals of magnesium and calcium phosphate. These crystals become part of the growing biofilm, which protects the bacteria from compounds used to coat catheters [
79]. Previous studies have shown that patients may develop UTIs within a few days of catheterization due to the presence of biofilm on urinary catheters [
26,
69]. Additionally, it has been found that patients who undergo catheterization for more than 28 days are at a high risk of developing CAUTIs [
17]. Biofilms formed in the initial stages of CAUTIs are often colonized by a single species, and subsequently, mixed communities develop, resulting in a thick biofilm that makes antibiotic therapy ineffective [
46]. CAUTIs are often caused by Gram-negative bacteria, such as
E. coli and
K. pneumoniae, originating from the patient’s perineal microbiota or from the hands of medical professionals [
80]. These bacteria can contaminate the urethra and migrate to the bladder, where they create biofilms that act as a reservoir of infection and promote antibiotic resistance. Catheters increase the risk of UTIs because they promote bacterial adherence by damaging the protective mucopolysaccharide layer of the uroepithelium, making it more vulnerable to bacterial invasion [
26]. Recent research has shown that enhancing care practices can reduce the incidence of CAUTIs and their associated negative outcomes, including higher costs, longer hospital stays, and increased mortality rates, particularly in critical care units [
81,
82].
4. Resistance of Bacteria in Biofilm
Antibiotic resistance may be significantly higher in uropathogens that reside in biofilms, up to 1000 times higher than in planktonic bacteria [
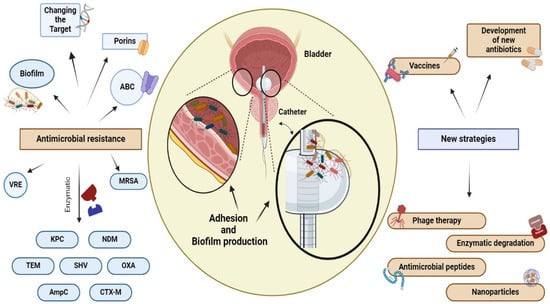
83]. Biofilms produced by uropathogens play a crucial role in antibiotic resistance through several mechanisms (
Figure 3). (1) Antibiotics diffuse inadequately within the biofilm due to the extracellular matrix, causing delayed penetration and reduced effectiveness; (2) bacteria within biofilms exhibit increased expression of efflux pumps compared to that of planktonic cells, which contributes to their resilient resistance to antibiotics; (3) the close cell-to-cell contact within biofilms facilitates the transmission of antibiotic resistance genes through HGT [
16,
83]. HGT facilitates the transfer of transposons and other mobile genetic elements between biofilm-forming cells, leading to the dissemination of resistance markers [
75]. These markers encode the secretion of antibiotic-inactivating enzymes and other virulence factors. (4) The presence of slow-growing persister cells that are intrinsically resistant to antibiotics represents a reservoir of surviving cells capable of rebuilding the biofilm population [
84]. The effectiveness of antibiotics is often limited by various mechanisms within a biofilm, which, in turn, drives antibiotic resistance, a topic that has been extensively investigated in many previous studies [
6,
16,
46].
Figura 3. Resistenza agli antibiotici e nuove strategie terapeutiche. I batteri possono sviluppare resistenza ai farmaci antimicrobici attraverso una varietà di meccanismi, compreso l’uso di enzimi idrolitici per inattivare il farmaco, l’alterazione del bersaglio del farmaco attraverso la mutazione, l’aumento dell’espressione dei trasportatori ABC per effondere il farmaco dalla cellula e la formazione di biofilm da uropatogeni sulla superficie dell'urotelio e su dispositivi fissi, come i cateteri. I meccanismi utilizzati da VAN e MRSA comportano l’alterazione del sito bersaglio, mentre TET, VIM e IMP sono associati alla degradazione enzimatica. Inoltre, i batteri uropatogeni impiegano vari metodi per diminuire l’efficacia degli agenti antimicrobici. Questi metodi includono la produzione di una parete cellulare ispessita, che rende difficile l'ingresso della vancomicina nella cellula (VISA), l'uso di pompe di efflusso e la modulazione dei canali delle porine. La presenza di biofilm aumenta la persistenza dei microrganismi e la loro resistenza antimicrobica. Gli antibiotici da soli potrebbero non essere sempre sufficienti per sradicare il biofilm. Pertanto, sono attualmente allo studio trattamenti alternativi come la fagoterapia, la degradazione enzimatica, l’uso di peptidi e nanoparticelle antimicrobiche, lo sviluppo di nuovi antibiotici e vaccini. NDM: New Delhi Metallo β-lattamasi, KPC: Klebsiella pneumoniae produttrice di carbapenemasi , TEM: β-lattamasi isolata da un'emocoltura di un paziente chiamato Temoniera in Grecia, SHV: variante sulfidrilica di TEM, OXA: oxacillinasi, AmpC: cefalosporinasi, CTX-M: cefotaximasi, VRE: Enterococcus resistente alla vancomicina , MRSA: Staphylococcus aureus resistente alla meticillina , ABC: cassetta legante l'ATP.