Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
In the oil and gas industry, the corrosion attributed to hydrogen sulfide (H2S) is one of the most significant challenges.
- oil and gas industry
- hydrogen sulfide corrosion
- corrosion mechanisms
1. Introduction
Pipelines play a crucial role in the oil and gas sector by facilitating the transportation of products to treatment facilities, storage depots, and refinery complexes [1]. Given that these pipelines transport valuable and hazardous substances, any potential failure carries significant financial and environmental consequences, including the risk of catastrophic economic losses and threats to human life [2]. Failures may arise from various factors, including corrosion (external, internal, and stress cracking), mechanical issues (such as material, design, and construction faults), third-party activities (accidental or intentional), operational problems (malfunctions, insufficiencies, disruptions of safeguarding systems, or operator errors), and natural phenomena (such as lightning strikes, floods, or land shifts) [3].
The distribution of failures over 15 years (1990–2005) is illustrated in Figure 1 [4]. Corrosion is the primary contributing factor, accounting for 46.6% of failures in natural gas pipelines and 70.7% in crude oil pipelines. A corrosion cost assessment conducted by a reputable oil and gas corporation revealed that in the fiscal year 2003, expenditures for corrosion amounted to approximately USD 900 million. The global expense attributed to corrosion in the oil and gas sector stands at approximately USD 60 billion. In the United States alone, documented corrosion-related costs in such industries reach USD 1.372 billion. Furthermore, considering the increasing demand for energy sourced from oil and gas and the associated concerns, worldwide corrosion expenses within the industry are expected to continue to rise [5]. Hence, there is a critical need for proactive risk assessments that balance cost-effectiveness and safety.
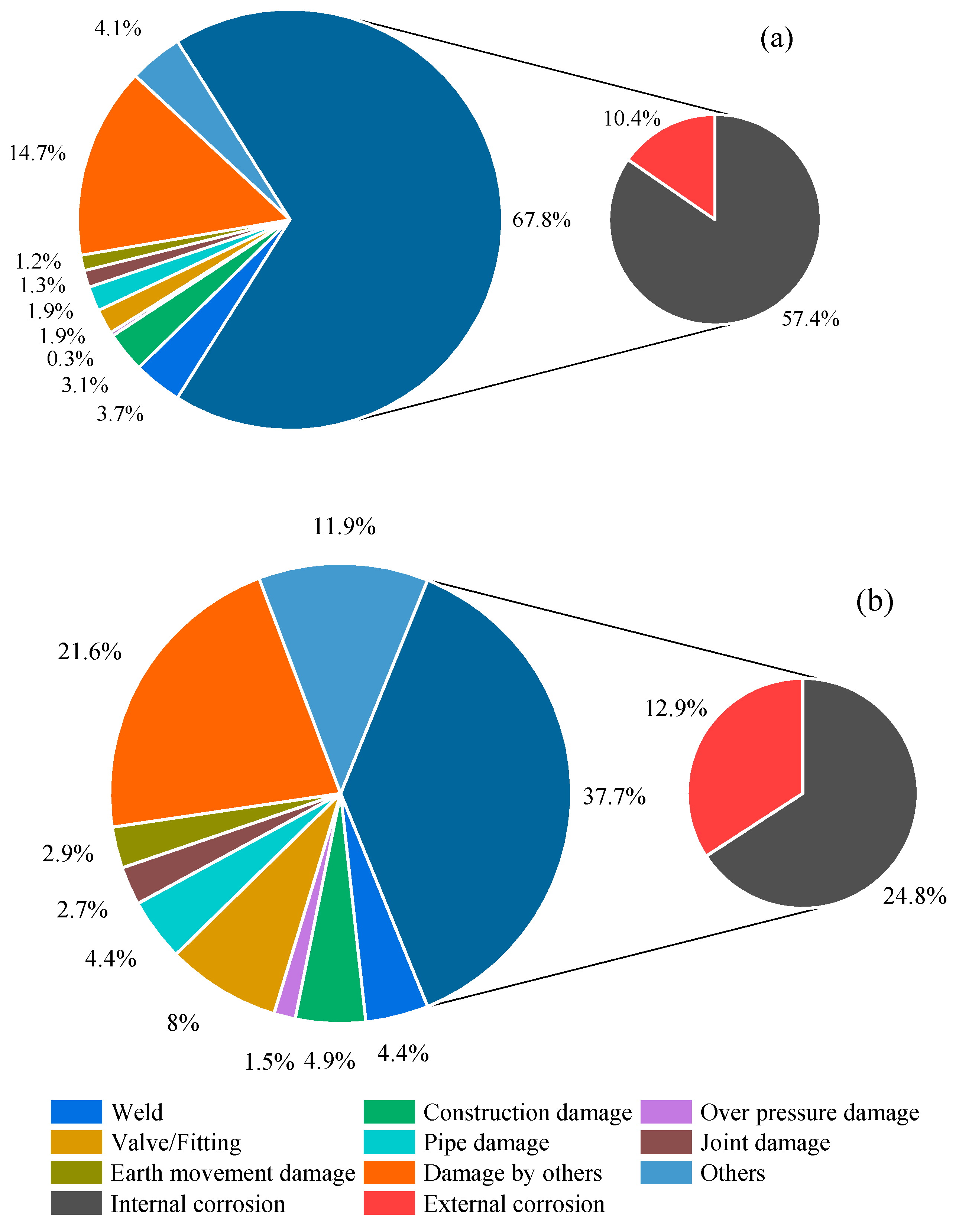
Figure 1. Pipeline incidents by cause (1990–2005): (a) crude oil (3826 incidents) and (b) natural gas pipelines (411 incidents).
Ensuring the integrity of pipelines is paramount for safe operations, environmental preservation, and the functionality of major production assets. Corrosion poses a serious threat, both externally and internally. External corrosion can result from factors such as oxygen and chloride in the external environment [6]. In contrast, internal corrosion may stem from substances such as hydrogen sulfide (H2S), carbon dioxide (CO2), and organic acids present in the production fluid. Unmonitored and uncontrolled pipeline corrosion can lead to leaks and catastrophic failures [7]. Internal corrosion has been a significant concern, constituting approximately 57.4% and 24.8% of corrosion failures in crude oil and natural gas pipelines, respectively (as indicated in Figure 1) [4]. Addressing internal corrosion is imperative for maintaining industry integrity and safety.
In the oil and gas sector, corrosion is typically categorized into two primary types: sweet and sour corrosion, prevalent in environments characterized by elevated partial pressures of H2S and CO2 (PH2S and PCO2). These particular forms of corrosion represent significant challenges within the industry. Corrosion is further categorized into three regimes based on the ratio of PCO2 to PH2S: sweet corrosion (PCO2/PH2S > 500), sweet–sour corrosion (PCO2/PH2S ranging from 20 to 500), and sour corrosion (PCO2/PH2S < 20:1) [8].
Critical factors influencing corrosion include PH2S and PCO2 levels, as well as temperature and pH values. These variables significantly affect the dissolution of corrosive gases, thereby influencing the rate and mechanism of corrosion product formation in sweet and sour environments. Temperature accelerates chemical reactions and increases gas solubility, impacting corrosion rates. pH levels determine environmental acidity or alkalinity, with low pH accelerating corrosion and high pH potentially triggering localized corrosion mechanisms. Dissolved CO2 and H2S gases generate corrosive acids in water, reacting with metal surfaces to form less protective compounds, thereby hastening corrosion. Sweet corrosion typically involves the creation of metal carbonates (MeCO3) [9], while sour corrosion involves various metal sulfide formations [10].
In the oil and gas sector, material failures resulting from corrosion in both sour and sweet environments pose various safety, economic, and environmental challenges. Figure 2 shows the relative contribution of various forms of corrosion failures throughout the 1970s [5]. Sour corrosion induced by H2S is identified as the primary cause of corrosion-related malfunctions in this industry, with its prevalence steadily escalating over time. Proactively addressing sour corrosion and instituting preventive measures are imperative for managing the associated risks in petroleum industries.
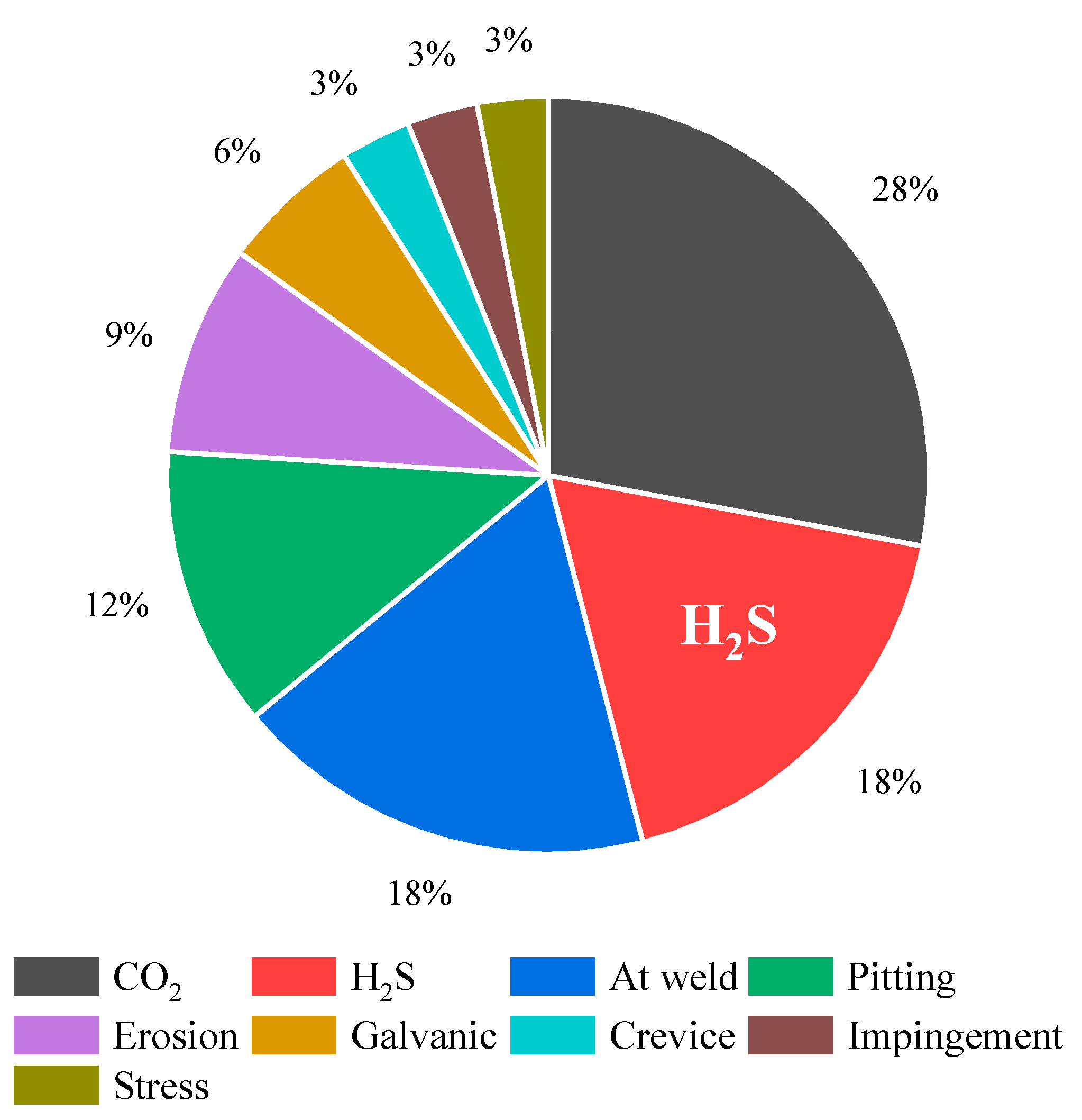
Figure 2. Primary contributors and their respective levels of influence on corrosion-related failures in the oil and gas industry (based on a 1970s study).
Managing and processing substances containing H2S pose significant challenges in the oil and gas sector. Understanding the intricacies of H2S corrosion is imperative, as it poses a substantial threat to equipment and infrastructure, elevating the risk of structural failure and potential accidents. This type of corrosion evidently diminishes the lifespan of equipment, necessitating costly maintenance or replacement endeavors. Moreover, it impedes operational efficiency, leading to decreased output and heightened energy consumption levels.
Comprehending and addressing the challenges posed by H2S corrosion within such industries yields noticeable advantages. Safety measures are strengthened by preventing breakdowns and maintaining equipment, and the possibility of accidents and environmental consequences is reduced. This strategy also prolongs the lifespan of equipment, diminishing the need for costly replacements and minimizing the downtime required for repairs. Additionally, it improves operational efficacy by guaranteeing effective and consistent procedures, reducing energy consumption, and reinforcing flow reliability.
Exploring areas for further investigation, including advanced coating technologies, new materials, electrochemical processes, and emerging technologies, is essential. The development of innovative approaches, such as continuous monitoring systems and predictive modeling, shows the potential to enhance precautionary measures. Applying advanced artificial intelligence and advanced analytics in management, prediction, and controlling corrosion is an emerging field that deserves further exploration.
2. H2S Corrosion in Refinery Operations
In refinery operations, H2S corrosion often arises due to sulfur-containing compounds present in natural gas derived from crude oil, gas wells, and oil well gas. These compounds include thiophenic compounds, sulfur alcohols, elemental sulfur, sulfur ethers, disulfides, and H2S. Additionally, more complex sulfides, such as metal sulfides, featuring multiple sulfur atoms or intricate molecular structures, may be encountered. These compounds exhibit a wider range of chemical properties and reactivities compared with simpler sulfides, posing potential challenges for corrosion management.
It is important to recognize the role of sulfate-reducing bacteria and microorganisms, which thrive in specific environments and contribute to H2S production. These bacteria aid in the breakdown of sulfur-containing compounds, releasing H2S as a byproduct. Moreover, sulfate-reducing bacteria can themselves decompose during formation, leading to H2S generation. Furthermore, the fluids used in oil and gas wells containing sulfonate can decompose at high temperatures, resulting in H2S formation. These various sources collectively contribute to the presence of H2S and the associated risk of corrosion in refinery processes.
2.1. Hydrogen Sulfide (H2S)
H2S is a toxic, corrosive, and flammable gas. It is colorless and easily distinguishable by its strong, pungent odor resembling ‘rotten eggs or cabbage’. When dissolved in water, H2S forms hydrosulfuric acid, resulting in a weakly acidic solution. In aqueous media, it readily dissociates into hydrosulfide, but not sulfide [11]. Due to its density being greater than that of air, H2S tends to accumulate in low-lying areas and depressions. Its ignition temperature is typically recognized as 518 °F (270 °C) [12]. Upon exposure to air, H2S undergoes rapid oxidation, producing sulfate and sulfur dioxide (SO2) due to the presence of oxidizing agents such as radicals in the air. This process results in a brief residence time of about 15 days for H2S in the atmosphere [12]. The lower and upper explosive limits of H2S are 4% and 44%, respectively. This indicates that H2S cannot combust in air at concentrations below 4% or above 44%. Combusting H2S yields SO2.
2.2. H2S Sources
In a refinery, H2S can originate from diverse sources during the processing of crude oil and other hydrocarbon products (Figure 3).
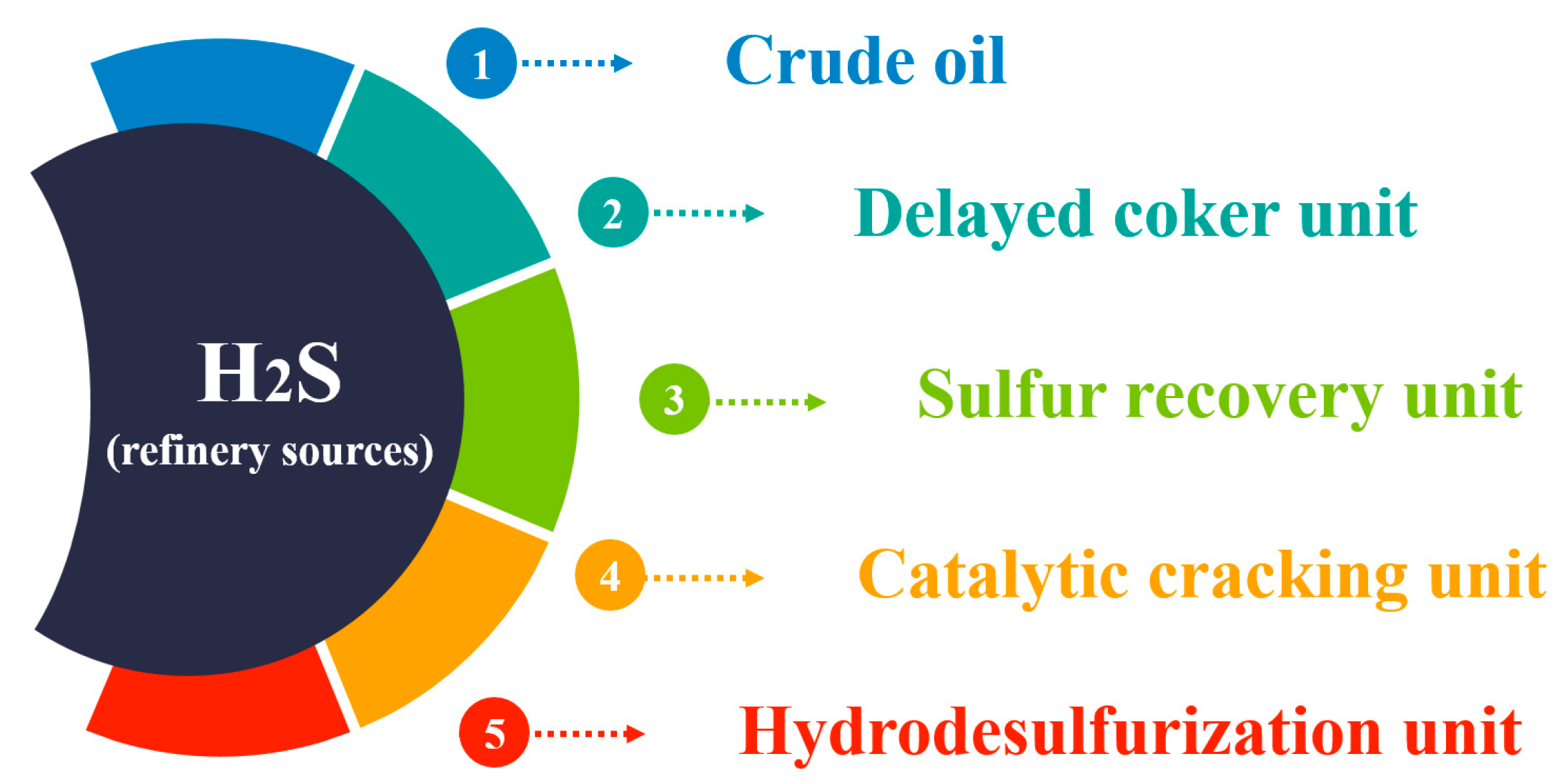
Figure 3. Main H2S sources in the refinery.
-
Crude oil serves as the primary source of H2S within refineries. During processing, naturally occurring sulfur compounds in crude oil release H2S gas [13]. Crude oil is typically categorized as “sweet” or “sour” based on its sulfur content, with sour crude oil containing higher levels of sulfur compounds, including H2S.
-
Various other sources within refinery facilities can also contribute to H2S production. Refineries utilize hydrodesulfurization units to efficiently remove sulfur from products such as jet fuel, diesel, and gasoline, converting sulfur compounds into H2S [14].
-
Catalytic reforming is a crucial process in refineries for converting low-octane hydrocarbons into high-octane gasoline blending components. This process can potentially generate H2S if sulfur-containing compounds are present in the feedstock [15].
-
Hydrotreating units utilize hydrogen gas to react with hydrocarbon streams, removing impurities such as sulfur and converting sulfur compounds into H2S [16].
-
Delayed coker units facilitate the conversion of heavy residuals into lighter products, including petroleum coke, which can also release H2S [17].
-
Sulfur recovery units (SRUs) in refineries extract elemental sulfur from sour gases produced during refining processes. H2S is typically converted into elemental sulfur or sulfuric acid in these units. However, incomplete conversion or operational inefficiencies can result in H2S emissions [18].
-
Sulfuric acid alkylation units, during the alkylation process, produce high-octane alkylate using sulfuric acid. While sulfuric acid primarily acts as a catalyst and is not consumed in the reaction, sulfur-containing impurities in the feedstock can lead to the formation of H2S as a byproduct [19].
-
Tank vents and storage facilities, especially those containing sulfur-containing products such as sour crude oil or intermediate products from desulfurization processes, may emit H2S when vented, particularly during filling or maintenance activities [20].
-
Wastewater treatment processes in refineries generate wastewater containing various contaminants, including sulfur compounds. During wastewater treatment, such as biological or chemical processes, H2S may be produced due to microbial activity or chemical reactions [21].
Figure 3 illustrates the various sources of H2S in a refinery, including crude oil processing and specific refinery units such as hydrodesulfurization, catalytic cracking, hydrotreating, and delayed coking.
2.3. H2S Corrosion Locations
Oil and gas reservoirs containing H2S concentrations exceeding 3 ppm by volume (ppmv) in the gas phase are referred to as sour hydrocarbon systems. The presence of H2S in these systems can lead to corrosion in downhole tubulars and surface infrastructure, known as sour corrosion. This corrosion is a primary factor in steel equipment failures within the oil and gas industry, particularly when H2S concentrations are notably higher than those of CO2 [22]. Sour corrosion presents significant challenges in refineries and other oil and gas facilities. Downhole tubulars are pipes and casings utilized in oil wells, extending from the surface into the wellbore, playing a crucial role in extracting and transporting oil and gas from underground reservoirs. Surface infrastructure encompasses all facilities and equipment above ground, including storage tanks, processing units, and other installations utilized in oil and gas operations. Various sections within a refinery are vulnerable to H2S corrosion, such as downstream equipment, hydrotreating units, catalytic reforming units, sour water strippers, amine units, flare systems, and heat exchangers (Figure 4).
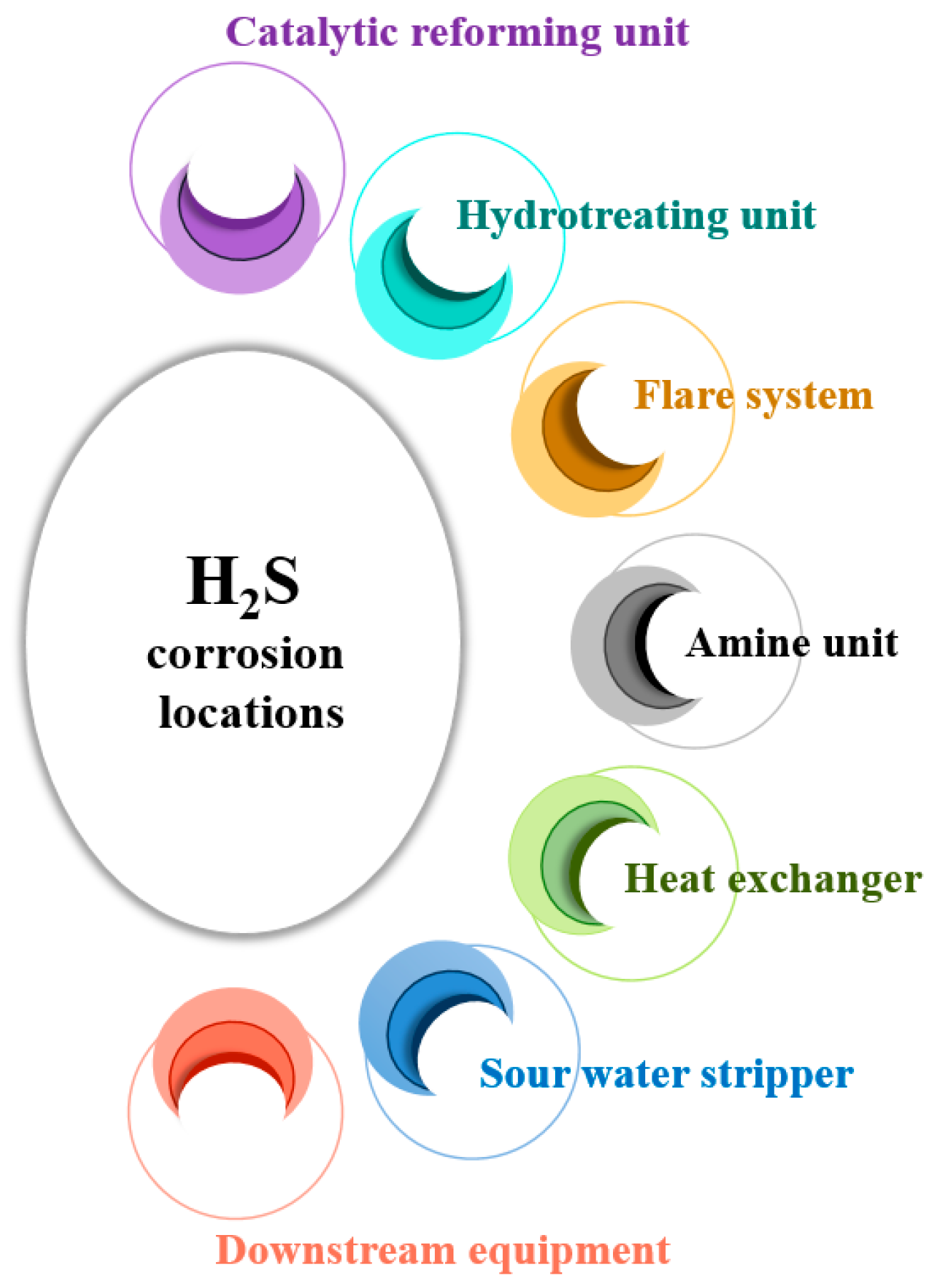
Figure 4. Specific locations susceptible to H2S corrosion in a refinery.
For example, Liu et al. [23] observed the development of dew point corrosion in the presence of H2S, leading to the fracturing and perforation of injection pipes. The rupture of the injection tube altered the flow rate and temperature characteristics of the overhead pipe. This corrosion primarily stemmed from the inadequacy of the material chosen for the inhibitor injection tube in this operational environment. Another investigation reported acid gas and sulfur leakage into the air from a refinery’s SRU [24]. This leakage happened because of heat exchanger malfunctions in the SRU, which were caused by the corrosion of mild steel equipment. This corrosion was linked to high levels of H2S in the system. The corrosion of steel by H2S resulted in the formation of iron sulfide, and the subsequent removal of this residue revealed pitting on the steel surface.
This entry is adapted from the peer-reviewed paper 10.3390/su16041661
References
- Muthukumar, N. Petroleum products transporting pipeline corrosion—A review. In The Role of Colloidal Systems in Environmental Protection; Fanun, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 527–571.
- Soomro, A.A.; Mokhtar, A.A.; Kurnia, J.C.; Lashari, N.; Lu, H.; Sambo, C. Integrity assessment of corroded oil and gas pipelines using machine learning: A systematic review. Eng. Fail. Anal. 2022, 131, 105810.
- Senouci, A.; Elabbasy, M.; Elwakil, E.; Abdrabou, B.; Zayed, T. A model for predicting failure of oil pipelines. Struct. Infrastruct. Eng. 2014, 10, 375–387.
- Pournara, A.-E. Structural Integrity of Steel Hydrocarbon Pipelines with Local Wall Distortions. Master’s Thesis, University of Thessaly, Volos, Greece, 2015.
- Obot, I.B.; Sorour, A.A.; Verma, C.; Al-Khaldi, T.A.; Rushaid, A.S. Key parameters affecting sweet and sour corrosion: Impact on corrosion risk assessment and inhibition. Eng. Fail. Anal. 2023, 145, 107008.
- Song, C.; Li, Y.; Wu, F.; Luo, J.; Li, L.; Li, G. Failure analysis of the crack and leakage of a crude oil pipeline under CO2-steam flooding. Processes 2023, 11, 1567.
- Zhao, W.; Zhang, T.; Wang, Y.; Qiao, J.; Wang, Z. Corrosion failure mechanism of associated gas transmission pipeline. Materials 2018, 11, 1935.
- Shi, X.; Zhang, Z.; Wu, L.; Li, X.; Zhang, Z. Corrosion law of metal pipeline in tahe oilfield and application of new materials. Coatings 2021, 11, 1269.
- Videm, K.; Koren, A. Corrosion, passivity, and pitting of carbon steel in aqueous solutions of HCO3−, CO2, and Cl−. Corrosion 1993, 49, 746–754.
- Obot, I.B.; Solomon, M.M.; Umoren, S.A.; Suleiman, R.; Elanany, M.; Alanazi, N.M.; Sorour, A.A. Progress in the development of sour corrosion inhibitors: Past, present, and future perspectives. J. Ind. Eng. Chem. 2019, 79, 1–18.
- Guidotti, T.L. Hydrogen sulfide intoxication. In Handbook of Clinical Neurology; Lotti, M., Bleecker, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 131, pp. 111–133.
- Ausma, T.; De Kok, L.J. Atmospheric H2S: Impact on plant functioning. Front. Plant Sci. 2019, 10, 743.
- Kraia, T.; Varvoutis, G.; Marnellos, G.E.; Konsolakis, M. Unveiling the role of in situ sulfidation and H2O excess on H2S decomposition to carbon-free H2 over cobalt/ceria catalysts. Catalysts 2023, 13, 504.
- Costa, C.; Cornacchia, M.; Pagliero, M.; Fabiano, B.; Vocciante, M.; Reverberi, A.P. Hydrogen sulfide adsorption by iron oxides and their polymer composites: A case-study application to biogas purification. Materials 2020, 13, 4725.
- Afzal, S.; Hussain, H.; Naz, M.Y.; Shukrullah, S.; Ahmad, I.; Irfan, M.; Mursal, S.N.F.; Legutko, S.; Kruszelnicka, I.; Ginter-Kramarczyk, D. Catalytic hydrogen evolution from H2S cracking over CrxZnS Catalyst in a cylindrical single-layered dielectric barrier discharge plasma reactor. Materials 2022, 15, 7426.
- Tian, R.; Xu, W.; Li, Y.; Tian, J.; Wu, L. Energy consumption analysis of a diesel hydrotreating unit using an aspen simulation. Processes 2022, 10, 2055.
- Magomedov, R.; Popova, A.; Maryutina, T.; Kadiev, K.M.; Khadzhiev, S. Current status and prospects of demetallization of heavy petroleum feedstock. Pet. Chem. 2015, 55, 423–443.
- Jagannath, A.; Ibrahim, S.; Raj, A. Heat integration in straight through sulfur recovery units to increase net high pressure steam production. Chem. Eng. Technol. 2021, 44, 164–173.
- Salah, H.B.; Nancarrow, P.; Al-Othman, A. Ionic liquid-assisted refinery processes—A review and industrial perspective. Fuel 2021, 302, 121195.
- Yang, R.; Zhirong, W.; Juncheng, J.; Shuoxun, S.; Peipei, S.; Yawei, L. Cause analysis and prevention measures of fire and explosion caused by sulfur corrosion. Eng. Fail. Anal. 2020, 108, 104342.
- Jiad, M.M.; Abbar, A.H. Petroleum refinery wastewater treatment using a novel combined electro-Fenton and photocatalytic process. J. Ind. Eng. Chem. 2024, 129, 634–655.
- Asadian, M.; Sabzi, M.; Anijdan, S.H.M. The effect of temperature, CO2, H2S gases and the resultant iron carbonate and iron sulfide compounds on the sour corrosion behaviour of ASTM A-106 steel for pipeline transportation. Int. J. Press. Vessels Pip. 2019, 171, 184–193.
- Liu, W.; Lyu, Y.; Duan, Z.; Li, W.; Yu, W. Investigation of corrosion sequence in the overhead pipeline of H2S stripper column through CFD models. Eng. Fail. Anal. 2022, 136, 106187.
- Lins, V.F.C.; Guimarães, E.M. Failure of a heat exchanger generated by an excess of SO2 and H2S in the sulfur recovery unit of a petroleum refinery. J. Loss. Prev. Process. Ind. 2007, 20, 91–97.
This entry is offline, you can click here to edit this entry!
