Cardiovascular diseases are the leading cause of death worldwide, and arterial hypertension is a recognized cardiovascular risk factor that is responsible for high morbidity and mortality. Arterial hypertension is the result of an inflammatory process that results in the remodeling and thickening of the vascular walls, which is associated with an immunological response. Previous studies have attempted to demonstrate the relationship between oral disease, inflammation, and the development of systemic diseases. The existence of an association between periodontitis and hypertension is a controversial issue because the underlying pathophysiological processes and inflammatory mechanisms common to both diseases are unknown.
- arterial hypertension
- periodontitis
- inflammation
- miRNAs
1. Introduction
2. Hypertension
3. Hypertension and Inflammation
4. Inflammation and Periodontitis
5. Hypertension, Periodontitis, and Inflammation
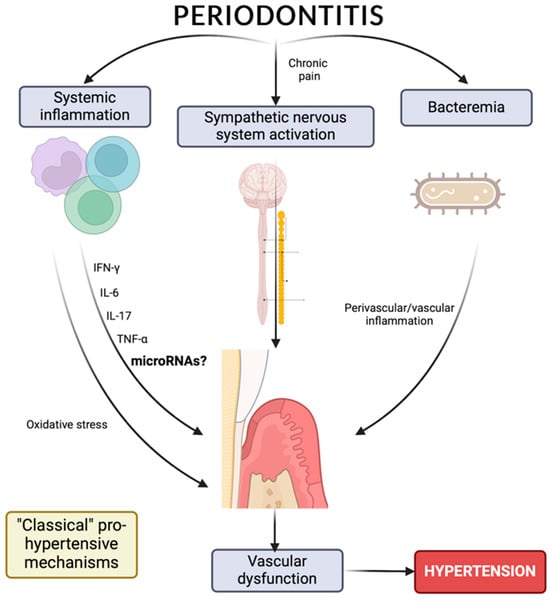
6. MicroRNAs as Risk Biomarkers for Hypertension and/or Periodontitis
| MicroRNAs | Model | Sample Size | Main Finding | Reference |
|---|---|---|---|---|
| miR-33a-5p, -144-3p | In vivo | Blood samples from 84 unrelated subjects (42 patients diagnosed with arterial hypertension and 42 normotensive subjects) | Hypertensive patients vs. control group: increased expression of miR-33a (p = 0.001) and miR-144 (p = 0.985), decreased expression of ABCA1 (p = 0.007) and ABCG1 (p = 0.550) transporters. | [76] |
| miR-153 | In vivo | Arteries from male normotensive Wistar rats and spontaneously hypertensive rats ranging from 12 to 16 in age | Increased expression of miR 153 in the arteries of spontaneously hypertensive rats (SHR) that exhibited decreased levels of Kv7.4. | [20] |
| miR-145 | In vivo and in vitro | Thoracic aortas from 10 26-week-old male spontaneously hypertensive rats and 10 age-matched normotensive male Wistar–Kyoto rats as control group Rat vascular endothelial cells isolated from the thoracic aortas |
miR-145 functions as a key mediator in the pathogenesis of hypertension through SLC7A1 targeting. | [77] |
| miR-126 | In vitro | HUVEC cells | Endothelial cells express miR-126, which inhibits VCAM-1 expression. | [78] |
| miR-146 | In vitro | THP-1, U937, HL-60, WEHI-3, 293/IL-1R/MD2/TLR4, BJAB, and Mono-Mac-6 cells | The role of miR-146 in the control of toll-like receptor and cytokine signaling through the downregulation of IL-1 kinase 1 and TNF receptor-associated factor 6 protein levels. | [79] |
| miR-296-5p, -let-7e, hcmv-miR-UL112. | In vivo | Whole blood from 194 hypertensive patients and 97 healthy volunteers | Twenty-seven differentially expressed miRNAs. Expressions of miR-296-5p, let-7e, and a human cytomegalovirus (HCMV)-encoded miRNA, hcmv-miR-UL112 were validated in plasma samples from 24 hypertensive patients and 22 control subjects. | [80] |
| miR-637 | In vitro | PC12 rat pheochromocytoma cells | ATP6V0A1 expression was affected by differential effects of miR-637, altering vacuolar pH and consequently CHGA processing and exocytotic secretion. | [81] |
| miR-1 | In vivo | Hearts isolated from Wistar rats divided into six groups: control, isoproterenol, ischaemia, ischaemia–propranolol, ischaemia–propranolol-miR-1, and ischaemia–AMO (n = 7 for each group) | The beta-adrenergic pathway can stimulate arrhythmogenic miR-1 expression, contributing to ischemic arrhythmogenesis, and beta-blockers produce their beneficial effects in part by downregulating miR-1. | [82] |
| miR-483-3p | In vivo and in vitro | Hearts obtained from age and gender matched transgenic mice over-expressing the human AT1R under the mouse α-MHC promoter and corresponding littermate non-transgenic mouse lines in C3H and C57BL/6 genetic backgrounds (n = 3 for each for group) Primary human aortic smooth muscle cells |
AT1R-regulated expression levels of angiotensin-1 and angiotensin-1 converting enzyme (ACE-1) proteins in VSMCs are specifically modulated by miR-483-3p. | [83] |
| miR-221, -222, -155 | In vitro | HUVEC cells | Increased expression of miR-155 and miR-221 in HUVEC and VSMC cells. The angiotensin II type 1 receptor (AT1R) is a target of miR-155 in HUVEC. Ets-1 and its downstream genes, including VCAM1, MCP1, and FLT1, were upregulated in angiotensin II-stimulated HUVECs, and this effect was partially reversed by overexpression of miR-155 and miR-221/2 | [84] |
| miR-208, -155 | In vivo | Aortas from 8-, 16-, and 24-week-old spontaneously hypertensive rats and 8-, 16-, and 24-week-old normotensive male Wistar–Kyoto rats as control group | The miR-155 level was negatively correlated with blood pressure (r = −0.525, p < 0.05). The expression of miR-208 in the aorta of hypertensive rats was negatively correlated with blood pressure (r = −0.400, p < 0.05) and age (r = −0.684, p < 0.0001). | [85] |
| MiR-21, -122, -637, -let-7e | In vivo | Plasma samples from 30 hypertension patients, 30 white coat hypertension patients, and 30 normotensive subjects | The expression levels of MiR-21, miR-122, miR-637, and let-7e were significantly increased in the group of hypertensive subjects compared to the group of normotensive subjects (p = 0.017, p = 0.022, p = 0.048 and p = 0.013, respectively). | [86] |
| miR-34a, -21, -126, -146a | In vivo | Plasma samples from 15 normotensive and 15 hypertensive subjects | Circulating expression of miR-34a was higher (~170%; p < 0.01) whereas expression of miR-21, miR-126, and miR-146a were markedly lower (~50%, ~55%, and ~55% respectively; p < 0.05) in the hypertensive versus normotensive. | [87] |
| miR-212, -132 | In vivo | Internal mammary artery with AngII receptor blockers (n = 16) and β-blockers (n = 9) from patients undergoing coronary artery by-pass graft surgery | miR-132 and miR-212 were upregulated in the heart, aortic wall, and kidney of rats with hypertension (159 ± 12 mm Hg) and cardiac hypertrophy after chronic Ang II infusion. In addition, activation of the endothelin receptor, another Gαq-coupled receptor, also increased miR-132 and miR-212. | [88] |
| miR-208b, -133a | In vivo | Blood and urine samples from 102 subjects with untreated newly diagnosed essential hypertension | miRNA-208b and miRNA-133a showed distinct profiles in peripheral blood cells isolated from untreated patients with newly diagnosed hypertension. Their gene expression levels revealed a strong correlation with urinary albumin excretion levels. | [89] |
| miR-25, -29a, -26b | In vivo | Blood samples from 104 acute Stanford type A aortic dissection + patients (of which 74 with hypertension and 30 without hypertension), and 103 age-matched acute Stanford type A aortic dissection individuals (of which 59 with hypertension and 44 without hypertension | 4-miRNA (miR-25, miR-29a, and miR-155) were significantly elevated, while miR-26b was decreased in AAAD+ serum samples compared to AAAD individuals), which may serve as a non-invasive biomarker for the diagnosis of AAAD, especially for subjects with hypertension. | [90] |
| miR-505 | In vivo and in vitro | Peripheral blood from 101 hypertensive patients and 91 healthy volunteers HUVEC cells |
The plasma level of hsa-miR-505 was significantly elevated in hypertensive patients. | [91] |
| miR-92a | In vivo | Plasma samples from 60 healthy volunteers with normal carotid intima-media thickness (nCIMT), 60 healthy volunteers with increased CIMT (iCIMT), 60 hypertensive patients with nCIMT and 60 hypertensive patients with iCIMT | miR-92a levels showed a significant positive correlation with mean 24-h systolic blood pressure (r = 0.807, p < 0.001), mean 24-h diastolic blood pressure (r = 0.649, p < 0.001), pulse pressure 24-h mean (PP) (r = 0.697, p < 0.001), 24-h daytime PP (r = 0.654, p < 0.001), 24-h nighttime PP (r = 0.573, p < 0.001), CIMT (r = 0.571, p < 0.001) and cfPWV (r = 0.601, p < 0.001). | [92] |
| miR-29a, -29b, -29c | In vivo | Whole blood from 54 patients with untreated hypertension and 30 healthy individuals | It was observed higher expression levels of miR-29a (p < 0.001), miR-29b (p < 0.001), and miR-29c (p < 0.001) in hypertensive patients compared to healthy control individuals. | [93] |
| miR-30e, -374b, -21 | - | Data obtained from Gene Expression Omnibus database | It was identified three crucial genes in the hypertensive kidney, such as COL12A1, ASPN, and SCN2A. ASPN could work in conjunction with COL12A1, and both could be targets for miR-21. SCN2A could be a new target for miR-30e and miR-374b. | [94] |
| miR-9, -126 | In vivo | Peripheral blood mononuclear cells of patients with essential hypertension (n = 60) and of healthy controls (n = 29) for comparison | Hypertensive patients showed significantly lower miR-9 (p < 0.001) and miR-126 (p < 0.001) expression levels compared to healthy controls. | [95] |
| miR-425, -155 | In vitro | Human embryonic stem cell-derived cardiomyocytes | The combination of miR-425 and miR-155 reduced NPPA expression to a greater extent than miR-425 or miR-155 alone, regardless of whether they separately also reduced NPPA expression. | [96] |
| miR-223 | In vivo | Samples from lean (n = 19) and obese (n = 19) patients | miR-223 mimics the downregulation of TLR4 expression in primary macrophages while downregulating the expression of FBXW7, a well-described suppressor of Toll-like receptor 4 (TLR4) signaling. | [97] |
| miR-1, -133a, -26b, -208b, -499, | In vivo | Peripheral blood mononuclear cells from 152 hypertensive patients and 30 healthy volunteers | Hypertensive patients showed significantly lower miR-133a expression levels (p < 0.001) and higher expression levels of miR-26b (p = 0.037), miR-1 (p = 0.019), miR-208b (p = 0.016), miR-499 (p = 0.033) and miR-21 (p = 0.002) compared to healthy controls. | [98] |
| miR-17-5p, -106a-5p, -106b-3p, -15a-5p, -15b-5p, -16-5p | In vivo | Case and control pairs (N = 15 pairs) selected from individuals with hypertension treatment | MiRs from the miR-17 and miR-15 families were downregulated in progressive chronic kidney disease with high blood pressure under hypertension treatment compared with appropriate controls. | [99] |
| miR-361-5p | In vivo | Whole blood samples from 50 paired hypertensive patients | Significant differences in hsa-miR-361-5p and hsa-miR-362-5p expression levels between samples from patients with salt-sensitive and salt-resistant hypertension (p = 0.023 and 0.049, respectively) | [100] |
| miR-34b | In vitro and in vivo | Vascular smooth muscle cells from the medial layer of the thoracic aorta collected from a total of 36 female spontaneously hypertensive and Wistar-Kyoto rats | The negative regulatory association between miR-34b and its target, CDK6, was confirmed, which has potential as a new therapeutic target in the treatment of hypertension. | [101] |
| miR-181a-5p, -27, -125a-5p, -27a-3p, -21-5p, -30a-5p, -98, -92, -22-3p, -100-5p, -99b-5p | In vitro | Human dermal microvascular endothelial cells | Significant suppression of ADM-encoded mRNA expression by endogenous miR-181a-5p, ATP2B1 by miR-27 family, FURIN by miR-125a-5p, FGF5 by let-7 family, GOSR2 by miR-27a-3p, JAG1 for miR-21-5p, SH2B3 for miR-30a-5p, miR-98, miR-181a-5p, and miR-125 family, TBX3 for miR-92 family, ADRA1B for miR-22-3p, ADRA2A by miR-30a-5p and miR-30e-5p, ADRA2B by miR-30e-5p, ADRB1 by the let-7 and miR-98 family, EDNRB by the miR-92 family, and NOX4 by the miR-92 family, miR -100-5p and miR-99b-5p (n = 3–9; p < 0.05 versus scrambled anti-miR). | [102] |
| MicroRNAs | Model | Sample Size | Main Finding | Reference |
|---|---|---|---|---|
| let-7a, -125b, -100, -21 | In vivo | Gingival tissue samples collected from 100 individuals with healthy gingiva and 100 chronic periodontitis patients | Expression analysis revealed that let-7a and miR-21 were upregulated, whereas miR-100, miR-125b, and LIN-28 were downregulated in chronic periodontitis patients relative to healthy individuals. They found that NF-κB was a common target among all four miRNAs. | [19] |
| let-7a, let-7c, -130a, miR301a, miR-520d and miR-548a, miR-181b, miR-19b, miR-23a, miR-30a, miR-let7a, miR-301a | In vivo | Normal healthy gingiva and diseased gingival tissues obtained from patients undergoing periodontal treatment | miR-let-7a, let-7c, miR-130a, miR301a, miR-520d, and miR-548a were more than 8-fold up-regulated compared to healthy gingiva. MiR-181b, miR-19b, miR-23a, miR-30a, miR-let7a, and miR-301a were successfully amplified and increased significantly more in periodontitis cases than in healthy subjects. | [103] |
| miR-1274b, -let-7b-5p, -24-3p, -19b-3p, -720, -126-3p, -17-3p, -21-3p. | In vivo | Gingival tissue samples from 9 nonsmoker individuals with chronic periodontitis and 9 nonsmoker individuals with aggressive periodontitis | No differences were observed in the expression profiles of miRNAs between aggressive periodontitis and chronic periodontitis (p > 0.05). The most expressed miRNAs in both groups were hsa-miR-1274b, hsa-let-7b-5p, hsa-miR-24-3p, hsa-miR-19b-3p, hsa-miR-720, hsa-miR-126-3p, hsa-miR-17-3p, and hsa-miR-21-3p. | [104] |
| miR-146a, -155 | In vivo | Gingival crevicular fluid from 24 healthy individuals with chronic periodontitis, 24 patients with chronic periodontitis in association with DM type 2, 24 healthy individuals with clinically healthy periodontium or 24 patients with clinically healthy periodontium in association with DM type 2 |
They revealed that miR-146a and miR-155 levels were significantly associated with periodontitis. | [105] |
| miR-17 | In vivo | Healthy human tooth samples collected from 8 individuals and teeth affected by periodontal disease collected from seven periodontics clinic patients diagnosed with chronic periodontitis | They found that inflammation resulted in an inhibition of miR-17 levels, which partly reversed the differentiation potential of mesenchymal stem cells (MSCs) isolated from periodontitis-affected periodontal ligament tissue (PDLSC). They confirmed that Smurf1 is a direct target of miR-17 in PDLSC. | [106] |
| miR-200b | In vivo | Gingival excess tissue sample was collected from obese and normal weight subjects | The miRNA profile of gingival tissue from obese patients with periodontitis, compared to normal weight patients, showed 13 upregulated and 22 downregulated miRNAs, among which miR-200b was validated by qRT-PCR for significantly increase in obesity. | [107] |
| miR-21-5p, -498, -548a-5p, -495-3p, -539-5p, -34c-3p, -7a-2-3p | In vitro | LPS-treated cells from primary human periodontal ligament isolated from explanted healthy periodontal ligament | It was identified 22 upregulated miRNAs and 28 downregulated miRNAs in the LPS-treated periodontal ligament. Seven upregulated (miR-21-5p, 498, 548a-5p) and downregulated (miR-495-3p, 539-5p, 34c-3p, and 7a-2-3p) miRNAs. | [108] |
| miR-302a-3p | In vitro | LPS-treated cells from human mandibular osteoblast-like cells and LPS-treated cells of immortalized normal oral keratinocyte | miR-302a-3p regulates RANKL expression in HMOB within the PGE2 -IFNγ regulatory network. | [109] |
| miR-543 | In vitro | Human periodontal ligament-derived stem cells | miR-543 was upregulated during osteogenic differentiation of human periodontal ligament-derived stem cells. Functional experiments showed that overexpression of miR-543 could enhance osteogenesis, while inhibiting miR-543 resulted in reduced formation of mineralized nodules. The ERBB2 transducer, 2 (TOB2) was identified as a target gene of miR-543. | [110] |
| miR-664a-3p, -501-5p, -21-3p | In vivo | Serum samples from 30 healthy patients without periodontitis and 30 patients with chronic periodontitis | The expression of hsa-miR-664a-3p, hsa-miR-501-5p, and has-miR-21-3p was higher in the periodontitis group than in the control group (p < 0.05). | [111] |
| miR-126*, -20a, -142-3p, -19a, -let-7f, -203, -17, -223, -146b, 146a, -155, -205 | In vivo | Gingival tissues obtained from 10 periodontitis patients and 10 healthy subjectshasHsa-miR-126*, hsa-miR-20a, hsa-miR-142-3p, hsa-miR-19a, hsa-lehasf, hsa-has-203, has-miR-17hassa-miR-223, hsa-miR-14has hsa-miR-146a, hsa-miR-155, and hsa-miR-205 showed differential expression levels in subjects with periodontitis in relation to healthy subjects. | [112] | |
| miR-128, -34a, -381, -15b, -211, -372, -656 | In vivo and in vitro | Gingival tissues from periodontitis patients and healthy subjects THP-1 cells and CA9-22 challenged with Porphyromonas gingivalis |
The gingival tissues of patients with periodontitis showed a higher expression of miRNA-128, miRNA-34a, and miRNA-381 and a decrease in the expression of miRNA-15b, miRNA-211, miRNA-372, and miRNA-656. | [113] |
| miR-150, -223, -200b, -379, -199a-5p, -214. | In vitro | Primary human gingival fibroblasts obtained from patient gingival connective tissue explants | The most overexpressed miRNAs (by >2.72 times) were hsa-miR-150, hsa-miR-223 and hsa-miR-200b, and the three most underexpressed miRNAs (by <0.39 times) were hsa-miR-379, hsa-miR-199a-5p and hsa-miR-214 in inflamed gums of patients with periodontitis. | [114] |
| miR-451, -223, -486-5p, -3917, -1246, -1260, -141, -1260b, -203, -210, -205 | - | Data obtained from Gene Expression Omnibus database | Four miRNAs (hsa-miR-451, hsa-miR-223, hsa-miR-486-5p, hsa-miR-3917) were significantly overexpressed and 7 (hsa-miR-1246, hsa-miR-1260, hsa-miR-141, hsa-miR-1260b, hsa-miR-203, hsa-miR-210, hsa-miR-205 *) were underexpressed by >2 times in diseased gums of patients with periodontitis versus healthy gums. | [115] |
| miR-200c | In vivo and in vitro | 12-week-old male Sprague Dawley rats microinjected with LPS-PG into the gingival sulcus Primary human gingival fibroblasts |
They confirmed that local treatment with miR-200c effectively protected alveolar bone resorption in a rat model of periodontitis, by reducing the distance between the cementum-enamel junction and the alveolar bone crest and the interroot space in the second upper molar. | [116] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms25041992
References
- Wermelt, J.A.; Schunkert, H. Management of arterial hypertension. Herz 2017, 42, 515–526.
- Solak, Y.; Afsar, B.; Vaziri, N.D.; Aslan, G.; Yalcin, C.E.; Covic, A.; Kanbay, M. Hypertension as an autoimmune and inflammatory disease. Hypertens. Res. 2016, 39, 567–573.
- Agita, A.; Alsagaff, M.T. Inflammation, Immunity, and Hypertension. Acta Med. Indones. 2017, 49, 158–165.
- Tsounis, D.; Bouras, G.; Giannopoulos, G.; Papadimitriou, C.; Alexopoulos, D.; Deftereos, S. Inflammation markers in essential hypertension. Med. Chem. 2014, 10, 672–681.
- Fischer, R.G.; Lira Junior, R.; Retamal-Valdes, B.; Figueiredo, L.C.; Malheiros, Z.; Stewart, B.; Feres, M. Periodontal disease and its impact on general health in Latin America. Section V: Treatment of periodontitis. Braz. Oral. Res. 2020, 34 (Suppl. S1), e026.
- Cardoso, E.M.; Reis, C.; Manzanares-Cespedes, M.C. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad. Med. 2018, 130, 98–104.
- Disease, G.B.D.; Injury, I.; Prevalence, C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259.
- Beck, J.D.; Offenbacher, S.; Williams, R.; Gibbs, P.; Garcia, R. Periodontitis: A risk factor for coronary heart disease? Ann. Periodontol. 1998, 3, 127–141.
- Danesh, J.; Whincup, P.; Walker, M.; Lennon, L.; Thomson, A.; Appleby, P.; Gallimore, J.R.; Pepys, M.B. Low grade inflammation and coronary heart disease: Prospective study and updated meta-analyses. BMJ 2000, 321, 199–204.
- Devaux, B.; Scholz, D.; Hirche, A.; Klovekorn, W.P.; Schaper, J. Upregulation of cell adhesion molecules and the presence of low grade inflammation in human chronic heart failure. Eur. Heart J. 1997, 18, 470–479.
- Samuels, M.A. Inflammation and neurological disease. Curr. Opin. Neurol. 2004, 17, 307–309.
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867.
- Lind, L. Circulating markers of inflammation and atherosclerosis. Atherosclerosis 2003, 169, 203–214.
- Kaptoge, S.; Seshasai, S.R.; Gao, P.; Freitag, D.F.; Butterworth, A.S.; Borglykke, A.; Di Angelantonio, E.; Gudnason, V.; Rumley, A.; Lowe, G.D.; et al. Inflammatory cytokines and risk of coronary heart disease: New prospective study and updated meta-analysis. Eur. Heart J. 2014, 35, 578–589.
- Engstrom, G.; Stavenow, L.; Hedblad, B.; Lind, P.; Tyden, P.; Janzon, L.; Lindgarde, F. Inflammation-sensitive plasma proteins and incidence of myocardial infarction in men with low cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2247–2251.
- Gurav, A.N. The implication of periodontitis in vascular endothelial dysfunction. Eur. J. Clin. Invest. 2014, 44, 1000–1009.
- Dinh, K.M.; Kaspersen, K.A.; Mikkelsen, S.; Pedersen, O.B.; Petersen, M.S.; Thorner, L.W.; Hjalgrim, H.; Rostgaard, K.; Ullum, H.; Erikstrup, C. Low-grade inflammation is negatively associated with physical Health-Related Quality of Life in healthy individuals: Results from The Danish Blood Donor Study (DBDS). PLoS ONE 2019, 14, e0214468.
- Munoz Aguilera, E.; Leira, Y.; Miro Catalina, Q.; Orlandi, M.; Czesnikiewicz-Guzik, M.; Guzik, T.J.; Hingorani, A.D.; Nart, J.; D’Aiuto, F. Is systemic inflammation a missing link between periodontitis and hypertension? Results from two large population-based surveys. J. Intern. Med. 2021, 289, 532–546.
- Venugopal, P.; Koshy, T.; Lavu, V.; Ranga Rao, S.; Ramasamy, S.; Hariharan, S.; Venkatesan, V. Differential expression of microRNAs let-7a, miR-125b, miR-100, and miR-21 and interaction with NF-kB pathway genes in periodontitis pathogenesis. J. Cell Physiol. 2018, 233, 5877–5884.
- Carr, G.; Barrese, V.; Stott, J.B.; Povstyan, O.V.; Jepps, T.A.; Figueiredo, H.B.; Zheng, D.; Jamshidi, Y.; Greenwood, I.A. MicroRNA-153 targeting of KCNQ4 contributes to vascular dysfunction in hypertension. Cardiovasc. Res. 2016, 112, 581–589.
- Almeida, M.I.; Reis, R.M.; Calin, G.A. MicroRNA history: Discovery, recent applications, and next frontiers. Mutat. Res. 2011, 717, 1–8.
- Lawes, C.M.; Vander Hoorn, S.; Rodgers, A.; International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet 2008, 371, 1513–1518.
- MINSAL Indicadores Básicos de Salud. Available online: https://repositoriodeis.minsal.cl/Deis/indicadores/IBS%202018.pdf (accessed on 26 January 2024).
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104.
- MINSAL Resultados I Encuesta de Salud. Available online: http://epi.minsal.cl/wp-content/uploads/2016/03/resumen-ejecutivo-vent.pdf (accessed on 26 January 2024).
- MINSAL II Encuesta de Calidad de Vida y Salud Chile. Available online: https://www.crececontigo.gob.cl/wp-content/uploads/2015/11/ENCAVI-2006.pdf (accessed on 26 January 2024).
- Gijon-Conde, T.; Gorostidi, M.; Camafort, M.; Abad-Cardiel, M.; Martin-Rioboo, E.; Morales-Olivas, F.; Vinyoles, E.; Armario, P.; Banegas, J.R.; Coca, A.; et al. Spanish Society of Hypertension position statement on the 2017 ACC/AHA hypertension guidelines. Hipertens. Riesgo Vasc. 2018, S1889-1837, 30033-3.
- Manfredi Carabetti, J.A. Endotelio, inflamación e hipertensión arterial. Rev. Urug. Cardiol. 2012, 27, 413–417.
- Wagner-Grau, P. Pathophysiology of arterial hypertension. An. Fac. Med. 2010, 71, 225–229.
- Savoia, C.; Schiffrin, E.L. Inflammation in hypertension. Curr. Opin. Nephrol. Hypertens. 2006, 15, 152–158.
- Roitt, I.M.; Brostoff, J.; Male, D.K. Immunology; Mosby: St. Louis, MI, USA, 1998.
- Male, D.C.B.; Cooke, A.; Owen, M. Cell troffic and inflammation. In Advanced Immunology, 2nd ed.; Lippincott: Philadelphia, CA, USA, 1991.
- Rosenberg, H.F.; Gallin, J.I. Chapter 37 Inflammation. In Fundamental Immunology, 6th ed.; Lippincott: Philadelphia, CA, USA, 2003.
- Oliveira, C.M.B.d.; Sakata, R.K.; Issy, A.M.; Gerola, L.R.; Salomão, R. Citocinas e dor. Rev. Bras. Anestesiol. 2011, 61, 260–265.
- Pastelin Hernandez, G.; Rosas Peralta, M. Inflammation in high blood pressure. Arch. Cardiol. Mex. 2007, 77 (Suppl. S4), 172–174.
- Leon-Pedroza, J.I.; Gonzalez-Tapia, L.A.; del Olmo-Gil, E.; Castellanos-Rodriguez, D.; Escobedo, G.; Gonzalez-Chavez, A. Low-grade systemic inflammation and the development of metabolic diseases: From the molecular evidence to the clinical practice. Cir. Cir. 2015, 83, 543–551.
- Vega, R.G.B. Inflamación. Rev. Fac. Med. UNAM 2008, 51, 220–222.
- Villalba-Herrera, E. Inflamación, I. Rev. Actual. Clínica 2014, 43, 2261–2265.
- Alonso-Rodriguez, D.; Moreno-Tellez, E.; Alarcon-Martinez, Y.; Pedroso-Filiberto, E. Reactive C protein as a marker of inflammation in patients with arterial hypertension. Rev. Med. Inst. Mex. Seguro Soc. 2011, 49, 345–347.
- Herrera Garza, E.H.; Herrera Garza, J.L.; Rodríguez González, H.; Treviño Treviño, A.; Ibarra Flores, M.; Torre Amione, G. Importance of Tumor Necrosis Factor-Alpha in the Pathogenesis of Heart Failure. Rev. Española Cardiol. 2002, 55, 61–66.
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667.
- Rodriguez Coyago, M.L.; Sanchez Temino, V.E. Periodontitis determining the onset and progression of Huntington’s disease: Review of the literature. Medwave 2015, 15, e6293.
- Carvajal, P. Enfermedades periodontales como un problema de salud pública: El desafío del nivel primario de atención en salud. Rev. Clínica Periodoncia Implantol. Rehabil. Oral. 2016, 9, 177–183.
- Moreno-Correa, S.C.-R.A. Molecular mechanisms involved in bone destruction in periodontitis. Literature review. Rev. Clin. Periodoncia Implantol. Rehabil. Oral. 2013, 6, 142–147.
- Nazir, M.A. Prevalence of periodontal disease, its association with systemic diseases and prevention. Int. J. Health Sci. 2017, 11, 72–80.
- Sanz, M.; D’Aiuto, F.; Deanfield, J.; Fernandez-Aviles, F. European workshop in periodontal health and cardiovascular disease--scientific evidence on the association between periodontal and cardiovascular diseases: A review of the literature. Eur. Heart J. Suppl. 2010, 12 (Suppl. B), B3–B12.
- Papapanou, P.N.; Susin, C. Periodontitis epidemiology: Is periodontitis under-recognized, over-diagnosed, or both? Periodontol. 2000 2017, 75, 45–51.
- MINSAL Protocolo de Referencia y Contrarreferencia para la Especialidad de Periodoncia. Available online: https://www.ssmn.cl/descargas/protocolos_referencia_contrareferencia/hospital_clinico_san_jose/odontologia/Protocolo_Periodoncia.pdf (accessed on 26 January 2024).
- Matos, M.G.; Israel, A.; Billet, E.; Garrido, M.d.R. Citocinas pro-inflamatorias en la enfermedad periodontal experimental: Efecto del valsartán. Rev. Fac. Farm. 2016, 79, 17–27.
- Monzón, J.; Acuña, M.; Caramello, C.; Sesín, J. Periodontitis como factor de riesgo de enfermedades cardiovasculares. Rev. Fac. Odontol. 2017, 10, 32–37.
- Carrillo de Albornoz Sainz, A.; García Kass, A.; Bascones Martínez, A. Papel de la IL-6 y TNF-a en la enfermedad periodontal. Av. Periodoncia Implantol. Oral. 2006, 18, 83–89.
- Botero, J.; Bedoya, E. Determinantes del diagnóstico periodontal. Rev. Clínica Periodoncia Implantol. Rehabil. Oral 2010, 3, 94–99.
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89 (Suppl. S1), S1–S8.
- Castro, C.E.; Koss, M.A.; Lopez, M.E. Biochemical markers of the periodontal ligament. Med. Oral. 2003, 8, 322–328.
- Socransky, S.S.; Haffajee, A.D. Microbial mechanisms in the pathogenesis of destructive periodontal diseases: A critical assessment. J. Periodontal Res. 1991, 26, 195–212.
- Contreras, A.; Ramírez, J. Relación entre Periodontitis y Enfermedad Cardiovascular. Rev. Clínica Periodoncia Implantol. Rehabil. Oral. 2009, 2, 91–97.
- Sanz, M.; Quirynen, M.; European Workshop in Periodontology Group A. Advances in the aetiology of periodontitis. Group A consensus report of the 5th European Workshop in Periodontology. J. Clin. Periodontol. 2005, 32 (Suppl. S6), 54–56.
- Heitz-Mayfield, L.J. Disease progression: Identification of high-risk groups and individuals for periodontitis. J. Clin. Periodontol. 2005, 32 (Suppl. S6), 196–209.
- Loos, B.G.; John, R.P.; Laine, M.L. Identification of genetic risk factors for periodontitis and possible mechanisms of action. J. Clin. Periodontol. 2005, 32 (Suppl. S6), 159–179.
- Page, R.C. The role of inflammatory mediators in the pathogenesis of periodontal disease. J. Periodontal Res. 1991, 26, 230–242.
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol. 2000 2017, 75, 7–23.
- Teeuw, W.J.; Laine, M.L.; Bizzarro, S.; Loos, B.G. A Lead ANRIL Polymorphism Is Associated with Elevated CRP Levels in Periodontitis: A Pilot Case-Control Study. PLoS ONE 2015, 10, e0137335.
- Gamonal, J.; Acevedo, A.; Bascones, A.; Jorge, O.; Silva, A. Levels of interleukin-1 beta, -8, and -10 and RANTES in gingival crevicular fluid and cell populations in adult periodontitis patients and the effect of periodontal treatment. J. Periodontol. 2000, 71, 1535–1545.
- Madianos, P.N.; Bobetsis, Y.A.; Kinane, D.F. Generation of inflammatory stimuli: How bacteria set up inflammatory responses in the gingiva. J. Clin. Periodontol. 2005, 32 (Suppl. S6), 57–71.
- Sima, C.; Glogauer, M. Diabetes mellitus and periodontal diseases. Curr. Diab. Rep. 2013, 13, 445–452.
- Forner, L.; Nielsen, C.H.; Bendtzen, K.; Larsen, T.; Holmstrup, P. Increased plasma levels of IL-6 in bacteremic periodontis patients after scaling. J. Clin. Periodontol. 2006, 33, 724–729.
- Czesnikiewicz-Guzik, M.; Osmenda, G.; Siedlinski, M.; Nosalski, R.; Pelka, P.; Nowakowski, D.; Wilk, G.; Mikolajczyk, T.P.; Schramm-Luc, A.; Furtak, A.; et al. Causal association between periodontitis and hypertension: Evidence from Mendelian randomization and a randomized controlled trial of non-surgical periodontal therapy. Eur. Heart J. 2019, 40, 3459–3470.
- Shrihari, T.G. Potential correlation between periodontitis and coronary heart disease—An overview. Gen. Dent. 2012, 60, 20–24.
- Del Pinto, R.; Ferri, C. Inflammation-Accelerated Senescence and the Cardiovascular System: Mechanisms and Perspectives. Int. J. Mol. Sci. 2018, 19, 3701.
- Martin-Cabezas, R.; Seelam, N.; Petit, C.; Agossa, K.; Gaertner, S.; Tenenbaum, H.; Davideau, J.L.; Huck, O. Association between periodontitis and arterial hypertension: A systematic review and meta-analysis. Am. Heart J. 2016, 180, 98–112.
- Cavagnari, B.M. Regulation of gene expression: How do epigenetic mechanisms work. Arch. Argent. Pediatr. 2012, 110, 132–136.
- Fernández-Sanjurjo, M.; Gonzalo-Calvo, D.d.; Díez-Robles, S.; Dávalos, A.; Iglesias-Gutiérrez, E. Circulating microRNA as regulators of the molecular response in exercise in healthy people. Arch. Med. Deport. 2016, 33, 394–403.
- de Gonzalo-Calvo, D.; Iglesias-Gutierrez, E.; Llorente-Cortes, V. Epigenetic Biomarkers and Cardiovascular Disease: Circulating MicroRNAs. Rev. Esp. Cardiol. 2017, 70, 763–769.
- Kaneto, C.M.; Nascimento, J.S.; Prado, M.; Mendonca, L.S.O. Circulating miRNAs as biomarkers in cardiovascular diseases. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2234–2243.
- Luan, X.; Zhou, X.; Naqvi, A.; Francis, M.; Foyle, D.; Nares, S.; Diekwisch, T.G.H. MicroRNAs and immunity in periodontal health and disease. Int. J. Oral. Sci. 2018, 10, 24.
- Huesca-Gomez, C.; Torres-Paz, Y.E.; Martinez-Alvarado, R.; Fuentevilla-Alvarez, G.; Del Valle-Mondragon, L.; Torres-Tamayo, M.; Soto, M.E.; Gamboa, R. Association between the transporters ABCA1/G1 and the expression of miR-33a/144 and the carotid intima media thickness in patients with arterial hypertension. Mol. Biol. Rep. 2020, 47, 1321–1329.
- Wang, Y.; Jin, L. miRNA-145 is associated with spontaneous hypertension by targeting SLC7A1. Exp. Ther. Med. 2018, 15, 548–552.
- Harris, T.A.; Yamakuchi, M.; Ferlito, M.; Mendell, J.T.; Lowenstein, C.J. MicroRNA-126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc. Natl. Acad. Sci. USA 2008, 105, 1516–1521.
- Taganov, K.D.; Boldin, M.P.; Chang, K.J.; Baltimore, D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. USA 2006, 103, 12481–12486.
- Li, S.; Zhu, J.; Zhang, W.; Chen, Y.; Zhang, K.; Popescu, L.M.; Ma, X.; Lau, W.B.; Rong, R.; Yu, X.; et al. Signature microRNA expression profile of essential hypertension and its novel link to human cytomegalovirus infection. Circulation 2011, 124, 175–184.
- Wei, Z.; Biswas, N.; Wang, L.; Courel, M.; Zhang, K.; Soler-Jover, A.; Taupenot, L.; O’Connor, D.T. A common genetic variant in the 3′-UTR of vacuolar H+-ATPase ATP6V0A1 creates a micro-RNA motif to alter chromogranin A processing and hypertension risk. Circ. Cardiovasc. Genet. 2011, 4, 381–389.
- Lu, Y.; Zhang, Y.; Shan, H.; Pan, Z.; Li, X.; Li, B.; Xu, C.; Zhang, B.; Zhang, F.; Dong, D.; et al. MicroRNA-1 downregulation by propranolol in a rat model of myocardial infarction: A new mechanism for ischaemic cardioprotection. Cardiovasc. Res. 2009, 84, 434–441.
- Kemp, J.R.; Unal, H.; Desnoyer, R.; Yue, H.; Bhatnagar, A.; Karnik, S.S. Angiotensin II-regulated microRNA 483-3p directly targets multiple components of the renin-angiotensin system. J. Mol. Cell Cardiol. 2014, 75, 25–39.
- Zhu, N.; Zhang, D.; Chen, S.; Liu, X.; Lin, L.; Huang, X.; Guo, Z.; Liu, J.; Wang, Y.; Yuan, W.; et al. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis 2011, 215, 286–293.
- Xu, C.C.; Han, W.Q.; Xiao, B.; Li, N.N.; Zhu, D.L.; Gao, P.J. Differential expression of microRNAs in the aorta of spontaneously hypertensive rats. Sheng Li Xue Bao 2008, 60, 553–560.
- Cengiz, M.; Karatas, O.F.; Koparir, E.; Yavuzer, S.; Ali, C.; Yavuzer, H.; Kirat, E.; Karter, Y.; Ozen, M. Differential expression of hypertension-associated microRNAs in the plasma of patients with white coat hypertension. Medicine 2015, 94, e693.
- Hijmans, J.G.; Diehl, K.J.; Bammert, T.D.; Kavlich, P.J.; Lincenberg, G.M.; Greiner, J.J.; Stauffer, B.L.; DeSouza, C.A. Association between hypertension and circulating vascular-related microRNAs. J. Hum. Hypertens. 2018, 32, 440–447.
- Eskildsen, T.V.; Jeppesen, P.L.; Schneider, M.; Nossent, A.Y.; Sandberg, M.B.; Hansen, P.B.; Jensen, C.H.; Hansen, M.L.; Marcussen, N.; Rasmussen, L.M.; et al. Angiotensin II regulates microRNA-132/-212 in hypertensive rats and humans. Int. J. Mol. Sci. 2013, 14, 11190–11207.
- Parthenakis, F.I.; Marketou, M.E.; Kontaraki, J.E.; Maragoudakis, F.; Maragkoudakis, S.; Nakou, H.; Roufas, K.; Patrianakos, A.; Chlouverakis, G.; Malliaraki, N.; et al. Comparative microRNA profiling in relation to urinary albumin excretion in newly diagnosed hypertensive patients. J. Hum. Hypertens. 2016, 30, 685–689.
- Xu, Z.; Wang, Q.; Pan, J.; Sheng, X.; Hou, D.; Chong, H.; Wei, Z.; Zheng, S.; Xue, Y.; Zhou, Q.; et al. Characterization of serum miRNAs as molecular biomarkers for acute Stanford type A aortic dissection diagnosis. Sci. Rep. 2017, 7, 13659.
- Yang, Q.; Jia, C.; Wang, P.; Xiong, M.; Cui, J.; Li, L.; Wang, W.; Wu, Q.; Chen, Y.; Zhang, T. MicroRNA-505 identified from patients with essential hypertension impairs endothelial cell migration and tube formation. Int. J. Cardiol. 2014, 177, 925–934.
- Huang, Y.; Tang, S.; Ji-Yan, C.; Huang, C.; Li, J.; Cai, A.P.; Feng, Y.Q. Circulating miR-92a expression level in patients with essential hypertension: A potential marker of atherosclerosis. J. Hum. Hypertens. 2017, 31, 200–205.
- Huang, Y.; Tang, S.; Huang, C.; Chen, J.; Li, J.; Cai, A.; Feng, Y. Circulating miRNA29 family expression levels in patients with essential hypertension as potential markers for left ventricular hypertrophy. Clin. Exp. Hypertens. 2017, 39, 119–125.
- Wang, G.; Wu, L.; Chen, Z.; Sun, J. Identification of crucial miRNAs and the targets in renal cortex of hypertensive patients by expression profiles. Ren. Fail. 2017, 39, 92–99.
- Kontaraki, J.E.; Marketou, M.E.; Zacharis, E.A.; Parthenakis, F.I.; Vardas, P.E. MicroRNA-9 and microRNA-126 expression levels in patients with essential hypertension: Potential markers of target-organ damage. J. Am. Soc. Hypertens. 2014, 8, 368–375.
- Vandenwijngaert, S.; Ledsky, C.D.; Agha, O.; Wu, C.; Hu, D.; Bagchi, A.; Domian, I.J.; Buys, E.S.; Newton-Cheh, C.; Bloch, D.B. MicroRNA-425 and microRNA-155 cooperatively regulate atrial natriuretic peptide expression and cGMP production. PLoS ONE 2018, 13, e0196697.
- Deiuliis, J.A.; Syed, R.; Duggineni, D.; Rutsky, J.; Rengasamy, P.; Zhang, J.; Huang, K.; Needleman, B.; Mikami, D.; Perry, K.; et al. Visceral Adipose MicroRNA 223 Is Upregulated in Human and Murine Obesity and Modulates the Inflammatory Phenotype of Macrophages. PLoS ONE 2016, 11, e0165962.
- Kontaraki, J.E.; Marketou, M.E.; Parthenakis, F.I.; Maragkoudakis, S.; Zacharis, E.A.; Petousis, S.; Kochiadakis, G.E.; Vardas, P.E. Hypertrophic and antihypertrophic microRNA levels in peripheral blood mononuclear cells and their relationship to left ventricular hypertrophy in patients with essential hypertension. J. Am. Soc. Hypertens. 2015, 9, 802–810.
- Nandakumar, P.; Tin, A.; Grove, M.L.; Ma, J.; Boerwinkle, E.; Coresh, J.; Chakravarti, A. MicroRNAs in the miR-17 and miR-15 families are downregulated in chronic kidney disease with hypertension. PLoS ONE 2017, 12, e0176734.
- Qi, H.; Liu, Z.; Liu, B.; Cao, H.; Sun, W.; Yan, Y.; Zhang, L. micro-RNA screening and prediction model construction for diagnosis of salt-sensitive essential hypertension. Medicine 2017, 96, e6417.
- Yang, F.; Li, H.; Du, Y.; Shi, Q.; Zhao, L. Downregulation of microRNA-34b is responsible for the elevation of blood pressure in spontaneously hypertensive rats. Mol. Med. Rep. 2017, 15, 1031–1036.
- Kriegel, A.J.; Baker, M.A.; Liu, Y.; Liu, P.; Cowley, A.W., Jr.; Liang, M. Endogenous microRNAs in human microvascular endothelial cells regulate mRNAs encoded by hypertension-related genes. Hypertension 2015, 66, 793–799.
- Lee, Y.H.; Na, H.S.; Jeong, S.Y.; Jeong, S.H.; Park, H.R.; Chung, J. Comparison of inflammatory microRNA expression in healthy and periodontitis tissues. Biocell 2011, 35, 43–49.
- Amaral, S.A.; Pereira, T.S.F.; Brito, J.A.R.; Cortelli, S.C.; Cortelli, J.R.; Gomez, R.S.; Costa, F.O.; Miranda Cota, L.O. Comparison of miRNA expression profiles in individuals with chronic or aggressive periodontitis. Oral. Dis. 2019, 25, 561–568.
- Radovic, N.; Nikolic Jakoba, N.; Petrovic, N.; Milosavljevic, A.; Brkovic, B.; Roganovic, J. MicroRNA-146a and microRNA-155 as novel crevicular fluid biomarkers for periodontitis in non-diabetic and type 2 diabetic patients. J. Clin. Periodontol. 2018, 45, 663–671.
- Liu, Y.; Liu, W.; Hu, C.; Xue, Z.; Wang, G.; Ding, B.; Luo, H.; Tang, L.; Kong, X.; Chen, X.; et al. MiR-17 modulates osteogenic differentiation through a coherent feed-forward loop in mesenchymal stem cells isolated from periodontal ligaments of patients with periodontitis. Stem Cells 2011, 29, 1804–1816.
- Kalea, A.Z.; Hoteit, R.; Suvan, J.; Lovering, R.C.; Palmen, J.; Cooper, J.A.; Khodiyar, V.K.; Harrington, Z.; Humphries, S.E.; D’Aiuto, F. Upregulation of gingival tissue miR-200b in obese periodontitis subjects. J. Dent. Res. 2015, 94 (Suppl. S3), 59S–69S.
- Du, A.; Zhao, S.; Wan, L.; Liu, T.; Peng, Z.; Zhou, Z.; Liao, Z.; Fang, H. MicroRNA expression profile of human periodontal ligament cells under the influence of Porphyromonas gingivalis LPS. J. Cell Mol. Med. 2016, 20, 1329–1338.
- Irwandi, R.A.; Khonsuphap, P.; Limlawan, P.; Vacharaksa, A. miR-302a-3p regulates RANKL expression in human mandibular osteoblast-like cells. J. Cell Biochem. 2018, 119, 4372–4381.
- Ge, Y.; Li, J.; Hao, Y.; Hu, Y.; Chen, D.; Wu, B.; Fang, F. MicroRNA-543 functions as an osteogenesis promoter in human periodontal ligament-derived stem cells by inhibiting transducer of ERBB2, 2. J. Periodontal Res. 2018, 53, 832–841.
- Yoneda, T.; Tomofuji, T.; Ekuni, D.; Azuma, T.; Maruyama, T.; Fujimori, K.; Sugiura, Y.; Morita, M. Serum microRNAs and chronic periodontitis: A case-control study. Arch. Oral. Biol. 2019, 101, 57–63.
- Xie, Y.F.; Shu, R.; Jiang, S.Y.; Liu, D.L.; Zhang, X.L. Comparison of microRNA profiles of human periodontal diseased and healthy gingival tissues. Int. J. Oral. Sci. 2011, 3, 125–134.
- Na, H.S.; Park, M.H.; Song, Y.R.; Kim, S.; Kim, H.J.; Lee, J.Y.; Choi, J.I.; Chung, J. Elevated MicroRNA-128 in Periodontitis Mitigates Tumor Necrosis Factor-alpha Response via p38 Signaling Pathway in Macrophages. J. Periodontol. 2016, 87, e173–e182.
- Ogata, Y.; Matsui, S.; Kato, A.; Zhou, L.; Nakayama, Y.; Takai, H. MicroRNA expression in inflamed and noninflamed gingival tissues from Japanese patients. J. Oral. Sci. 2014, 56, 253–260.
- Stoecklin-Wasmer, C.; Guarnieri, P.; Celenti, R.; Demmer, R.T.; Kebschull, M.; Papapanou, P.N. MicroRNAs and their target genes in gingival tissues. J. Dent. Res. 2012, 91, 934–940.
- Akkouch, A.; Zhu, M.; Romero-Bustillos, M.; Eliason, S.; Qian, F.; Salem, A.K.; Amendt, B.A.; Hong, L. MicroRNA-200c Attenuates Periodontitis by Modulating Proinflammatory and Osteoclastogenic Mediators. Stem Cells Dev. 2019, 28, 1026–1036.
