India continues to grapple with a significant burden of HIV infections. Despite notable progress in prevention and treatment efforts, multiple challenges, such as high-risk populations, inadequate testing facilities, and limited access to healthcare in remote areas, persist. Though the Government of India offers HIV-1 plasma viral load testing at various medical centers, aiding treatment decisions and monitoring antiretroviral therapy effectiveness, enhancing care for individuals living with HIV under the National AIDS Control Program (NACP), the nation’s large population and diverse demographics further complicate its outreach and response. Hence, strategic interventions and alternative methods of testing remain crucial to curbing HIV transmission and improving the quality of life for those affected. Dried blood spot (DBS) sampling has emerged as a convenient and cost-effective alternative for HIV-1 viral load testing, revolutionizing the landscape of diagnostic and monitoring strategies for HIV infection. Though the plasma-based viral load remains the gold standard for monitoring HIV-1, DBS-based HIV-1 viral load testing holds immense promise for improving access to care, particularly in resource-limited settings where traditional plasma-based methods may be logistically challenging. DBS entails the collection of a small volume of blood onto filter paper, followed by drying and storage. This approach offers numerous advantages, including simplified sample collection, transportation, and storage, reducing the need for cold-chain logistics.
1. Conventional Method of HIV-1 Viral Load Testing
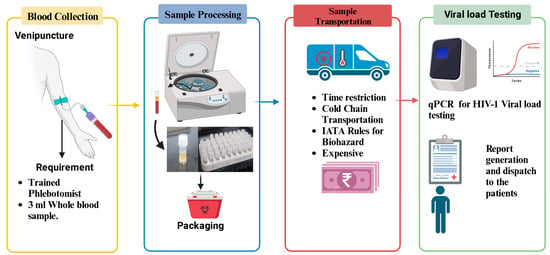
The conventional method of HIV-1 viral load testing primarily relies on quantifying the amount of HIV-1 RNA in a patient’s plasma, providing a crucial indicator of the viral replication activity within the body (Figure 1). This process involves the collection of a venous blood sample from the patient. The collected blood is then transported under controlled conditions to a diagnostic laboratory, where specialized equipment, such as real-time polymerase chain reaction (PCR) machines, is utilized. PCR amplifies the viral RNA in the sample, enabling precise quantification. The process requires technical expertise and an established infrastructure of laboratories. Moreover, cold-chain logistics are crucial to maintaining the integrity of the plasma samples during transportation, adding to the complexity and cost of the conventional method. Despite being the established gold standard, the conventional approach has limitations, especially in resource-limited settings, due to its demanding infrastructure requirements, cost, and dependence on a well-developed healthcare system.
Figure 1. Flowchart illustrating the process of HIV-1 plasma viral load testing: This figure provides a detailed flowchart outlining the sequential steps involved in the conventional method of plasma HIV-1 viral load testing.
2. The Potential of Dried Blood Spot Analysis as a Convenient and Cost-Effective Alternative for HIV-1 Viral Load Measurement
Historically, the concept of dried blood spots as a sample type originated around the end of World War II. The DBS technique primarily employs filter paper to absorb blood samples, which are subsequently utilized for laboratory testing [
20]. Robert Guthrie notably pioneered the application of DBS for phenylketonuria screening in 1960. Today, DBS finds wide-ranging utility in serological diagnosis, encompassing diseases like AIDS, hepatitis, trypanosomiasis, hepatic amebiasis, congenital rubella, and more [
21].
In the early 2000s, there was a growing interest in employing DBS for therapeutic monitoring of HIV infection, particularly in resource-limited settings where access to nearby laboratories remains a substantial challenge due to constraints in human and financial resources. DBS emerges as a cost-effective alternative, notably in remote, challenging-to-reach rural areas where plasma specimen preparation and transport are hindered by costly cold-chain requirements and a scarcity of trained personnel for venipuncture and plasma separation. In light of this, recent WHO guidelines for antiretroviral therapy advocate for DBS as a viable option to enhance access to viral load monitoring for HIV, HBV, and HCV diagnosis [
22].
Numerous studies have consistently demonstrated a robust correlation between DBS viral load assessments and plasma-based viral load testing [
23,
24]. These investigations have underscored the exceptional sensitivity and specificity of DBS specimens for detecting HIV-1 viral loads. DBS analysis presents compelling advantages as an accessible and cost-effective alternative for quantifying HIV-1 viral loads. It eliminates the need for intrusive venipuncture, streamlining sample collection and enabling self-sampling, especially in remote or resource-constrained regions where healthcare accessibility and transportation pose challenges. The dried form of DBS samples ensures convenient storage, transport, and reduced biohazard risks compared to liquid samples. Furthermore, it reduces the demand for complex cold-chain logistics, resulting in substantial cost savings and widened testing availability.
DBS analysis also demands smaller blood volumes, mitigating patient discomfort and facilitating pediatric testing. This approach proves particularly beneficial in regions where traditional plasma-based techniques face hurdles due to infrastructure limitations. DBS samples can be efficiently collected, shipped, and analyzed, promoting timely diagnosis, treatment monitoring, and therapy adjustments. These advantages firmly establish DBS analysis as a transformative tool, addressing gaps in HIV-1 viral load monitoring and advancing global HIV control and healthcare objectives.
3. Advantages of DBS over Plasma Specimen
Dried blood spots offer distinct advantages over plasma specimens, primarily in terms of their simplicity and cost-effectiveness in the collection and transportation process. To obtain a DBS sample, a simple finger prick is all that is needed; it does not require the presence of a trained phlebotomist for blood collection. This convenience extends to the possibility of collecting DBS samples outside clinical settings, reducing the burden on patients who would otherwise have to visit a clinic.
Following collection, DBS cards are left to air-dry overnight, then packaged and sent to a testing facility for analysis at room temperature. Unlike other methods, DBS specimens can be shipped to public health laboratories through standard mail, courier services, or express delivery as exempted specimens under the regulations issued by the International Air Transporter Association (IATA), the World Health Organization, and the International Civil Aviation Organization. DBS eliminates the need for expensive cold-chain transport and cryopreservation, as detailed in the CDC guidelines [
25].
This streamlined approach significantly simplifies the pre-analytical phase of sample collection. Moreover, because DBS samples don’t require a cold-chain system to maintain their integrity, the overall cost of viral load testing in research studies is substantially reduced, making it a highly cost-effective option.
Performance assessment and implementation of DBS specimens for HIV-1 viral load testing have been the subject of numerous studies. The journey began with the WHO 2013 consolidated guidelines on antiretroviral drug use, which emphasized the need for higher detection thresholds when using DBS specimens for viral load testing, given uncertainties about their accuracy, especially below 1000 copies/mL.
In 2016, a study on DBS for HIV-1 viral load assays reported approximately 80% sensitivity and 90% specificity [
26]. Misclassification rates were slightly lower in high viral load cases but ranged from 20.3% to 23.6% in low viral load cases. A 2017 study comparing DBS-fingerprint and DBS-venous methods found a sensitivity of 93% and a specificity of 95% for both DBS approaches [
27]. In another 2017 study using Abbott and Roche platforms, DBS showed sensitivity and specificity of 93.9% and 88.0%, respectively, for Abbott platforms. Sensitivity was 100% for CAP/CTM, albeit with slightly lower specificity compared to Abbott [
28]. A 2017 field evaluation in Kenya, a resource-limited setting, reported DBS performance at a threshold of ≥1000 copies/mL, with sensitivity ≥88.1% and specificity ≥93.1% [
29]. A WHO pre-qualification study in 2018 reported DBS sensitivity of 76.0% and specificity of 89.7% at the 1000 RNA copies/mL threshold [
21,
30]. A cross sectional study comparing DBS HIV VL with plasma values on the Hologic Panther platform demonstrated a positive correlation of 0.96 [
23]. A meta-analysis by the WHO and CDC in 2022 indicated that four out of six technologies using DBS samples for viral load testing achieved sensitivity and specificity above 83% at a treatment failure threshold of 1000 copies/mL. Notably, Abbott Real Time HIV-1, BioMerieux NucliSENS HIV-1, Roche COBAS TaqMan with FVE protocol, and Siemens VERSANT HIV-1 RNA performed exceptionally well under this threshold [
11]. Furthermore, studies have highlighted the potential for enhanced DBS performance through the adoption of proper extraction protocols [
24,
31,
32,
33,
34]. In summary, ongoing technological advancements are continually improving the reliability of DBS specimens in HIV-1 viral load testing (
Table 1).
4. Feasibility Study Results of Implementation of DBS for Viral Load Testing in Different Setups
The feasibility of implementing the DBS specimen for expanding viral load programs has been rigorously examined by scientists worldwide, and the results suggest promising potential for its practical application in the field. Numerous studies conducted across various platforms have consistently highlighted the complementary nature of DBS as a valuable adjunct to plasma samples for improving access to viral load monitoring [
11,
12]. This complementarity is especially pronounced in resource-limited rural settings, where the advantages of DBS, such as its economical transport mechanism and ease of handling, become particularly significant. While DBS stands out as a crucial complementary tool, it is essential to recognize that, in resource-limited settings, it is often suggested as a primary approach due to its cost-effectiveness, simplified logistics, and potential to address challenges associated with traditional plasma-based methods. In India, where the HIV epidemic is primarily concentrated among high-risk groups and key populations like female sex workers, men who have sex with men, transgender individuals, and injecting drug users, the NACO initiated a study in 2012. This study identified the essential need for implementing DBS as an alternative sample type in India, taking into account its suitability for HIV-1 viral load testing [
32]. DBS has been validated for HIV-1 viral load and HIV-1 DBS qualitative assays on various platforms, including the Indian Council of Medical Research-National AIDS Research Institute (ICMR-NARI) in India. A recent review published in 2022 by ICMR summarized the usage of dried blood specimens for screening sexually transmitted infections (STIs) and emphasized its role in expanding the HIV-1 viral load program to achieve the global 95-95-95 target in India [
35]. In our proposed study, the research team has devised a protocol to assess the feasibility of alternative methods for viral load expansion in India, with a specific focus on the implementation capabilities of DBS in HIV-1 viral load expansion in the country [
36]. A feasibility study conducted in Thailand in August 2021 reported a correlation coefficient of 0.62 between HIV viral load measured from plasma and DBS, with a mean difference of 0.02 (SD: 1.06) log10 IU/mL. At the threshold defining treatment failure (1000 copies/mL), the sensitivity of DBS HIV-1 RNA detection was 86% (95% CI, 74–94%). This study also demonstrated the high stability of HIV RNA in DBS after 2 to 4 days of shipping, compared to HIV RNA before shipping, even under varying temperature and humidity conditions [
24]. A Vietnam feasibility study on dried blood spots for routine viral load monitoring, particularly to reach hard-to-reach populations, showed comparable results with plasma during virological failure and highlighted the usefulness of DBS in monitoring hard-to-reach patients during HIV care [
13].
The feasibility of DBS for viral load testing has also been explored in the United States, where at-home DBS collection and longitudinal VL monitoring among low-ART-adherence MSM living with HIV showed promise. This mode of viral testing improved viral suppression in this group by simplifying sample collection and transport procedures for viral load testing [
37]. Additionally, an African study reviewing progress in viral load expansion identified limitations related to transport conditions and turnaround times for specimen transfer to VL testing facilities. It discussed the need for implementing DBS and point-of-care (POC) testing, where applicable [
24].
In a study conducted across seven PEPFAR-supported countries (Côte d’Ivoire, Kenya, Malawi, Mozambique, Namibia, South Africa, and Uganda) with a high pediatric HIV burden, an increasing proportion of VL tests were observed using dried blood spots, and lower sample rejection rates were noted for DBS compared to plasma [
38]. A recent article published in AIDS Patient Care and STDs in 2023 asserted that DBS is a suitable and cost-effective tool for viral load monitoring when an ideal implementation protocol is followed to increase viral load testing in the population [
39].
5. Principle of DBS-Based HIV-1 Viral Load Testing
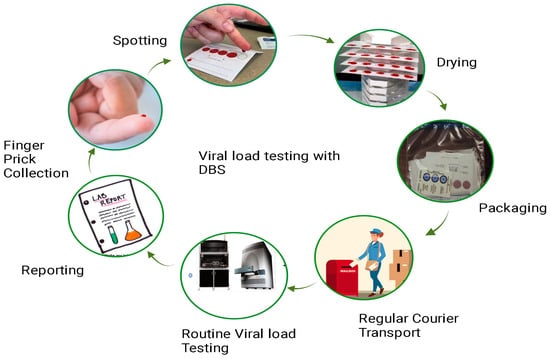
The principle of DBS-based HIV-1 viral load testing revolves around the collection, drying, and subsequent analysis of a small volume of blood from an individual living with HIV (Figure 2). This method eliminates the need for immediate sample processing and enables easy transportation and storage.
Figure 2. Workflow of dried blood spot viral load testing: This figure presents a detailed workflow illustrating the process of conducting viral load testing using DBS samples. The workflow begins with the collection of a small blood sample, typically obtained through a fingerstick. The DBS cards, on which the blood is applied, are then allowed to dry thoroughly. Subsequently, the dried blood spot cards are packaged and transported to the testing facility. Within the laboratory, the DBS samples undergo extraction of RNA and are subjected to molecular assays, such as polymerase chain reaction (PCR), to quantify HIV-1 RNA levels. The final steps involve result interpretation, contributing to a comprehensive understanding of the DBS viral load testing procedure.
Methodology for Working with DBS
Dried blood spots are specimens prepared by collecting the blood spots from a finger or heel prick and spotting them directly onto a filter paper, which is then used for testing in the laboratory as a sample. The detailed process involves the following steps:
-
Blood Collection: A small volume of blood, usually obtained through a finger prick, is collected onto a filter paper card specifically designed for DBS collection. The filter paper absorbs the blood and preserves it.
-
Drying: The blood-soaked filter paper is allowed to air-dry completely, usually at room temperature. This step stabilizes the blood components on the filter paper, preventing degradation of the biological material.
-
Sample transport and storage: Once dried, the DBS card can be stored at room temperature for an extended period. This eliminates the need for immediate cold-chain transportation and storage, making it particularly advantageous in resource-limited or remote settings.
-
Sample extraction and analysis: To perform HIV-1 viral load testing, a small disc or punch is cut from the dried blood spot on the filter paper. This disc is then extracted to obtain the genetic material (RNA) of the virus. The extracted RNA is then subjected to nucleic acid amplification techniques, such as reverse transcription polymerase chain reaction (RT-PCR), to quantify the amount of HIV-1 viral RNA present in the blood.
The viral load determined through DBS analysis directly reflects the quantity of the virus present in an individual’s bloodstream. This metric holds immense significance as it serves as a critical gauge for assessing HIV progression and gauging the effectiveness of antiretroviral therapy. HIV-1 viral load testing through DBS analysis presents a streamlined, cost-efficient, and pragmatic means of monitoring the HIV-1 viral load, especially in regions grappling with limited resources or intricate logistical challenges.
This entry is adapted from the peer-reviewed paper 10.3390/healthcare12040413