Legumes are a source of important secondary metabolites including carotenoids, and they play a significant role in food and diet diversification and ecosystem protection. Carotenoids are the second-most abundant naturally occurring pigments on earth, synthesized by plants, which fulfill important physiological functions. The main carotenoid pigments found in the photosystems of plants include α-Carotene and β-carotene, which are further hydroxylated to produce xanthophylls (e.g., lutein and zeaxanthin). The crucial roles of carotenoids and their metabolites in photooxidative protection and photosynthesis, not to mention nutrition, vision, and cellular differentiation, make them an important class of biological pigments. In cowpeas, carotenoids are mainly present in seeds, leaves, and pods, which contribute to the antioxidant properties of this legume. Increasing the content of carotenoids in cowpeas will contribute to food and nutrition security in the tropics.
1. Breeding for Increased Carotenoid Content in Cowpea
In recent decades, biofortification has gained importance as one of the most sustainable ways to supply micronutrient-rich foods for alleviating hidden hunger and malnutrition worldwide [
107,
108]. In regard to carotenoids, most of the research efforts have focused on increasing the carotenoid precursors of vitamin A (provitamin-A carotenoids), such as β-carotene, α-carotene, and β-cryptoxanthin. Consequently, there have been significant advances in breeding for provitamin-A carotenoid varieties in some major crops, including maize, cassava, and sweet potato [
8,
109]; however, the potential of the legume grain crops including cowpea is still untapped. It is, thus, important to leverage the lessons and progress in other crops, in other to define an effective approach (
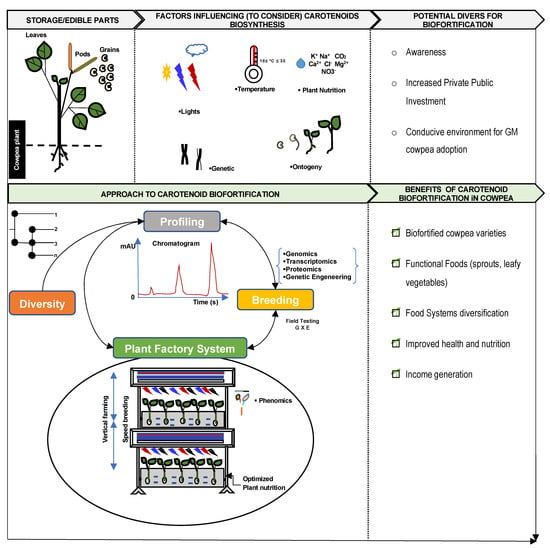
Figure 1) for the biofortification of carotenoids in cowpea.
Figure 1. Road map for biofortification of carotenoids in cowpea.
The extent of genetic gain in breeding cowpea for enhanced carotenoid content depends on the knowledge of the genetic diversity for the trait. The screening and evaluation of crop diversity is the first and most important step in breeding for carotenoid-biofortified varieties [
110]. The literature survey showed that information on the diversity of carotenoid content in cowpea is still very scant. Nonetheless, there have been extensive efforts on the collection and conservation of cowpea genetic resources, with large germplasm collections maintained at different genes banks, which can be used as working materials: IITA (15,003 accessions), the United States Department of Agriculture (USDA)–Genetic Resources Information Network (7737 accessions), and the University of California, Riverside (UCR), collections of 6000 accessions [
111,
112]. To save time and resources, the screening and evaluation can be narrowed down to the established mini-core collections from these various gene banks, which capture most of the existing diversity in the crop. The mini-core collections include 298 accessions from the IITA collections [
112]; 368 accessions from the UCR collections [
113]; and 369 accessions from the USDA cowpea germplasm [
114].
The assessment of the genetic diversity for carotenoid content should integrate both biochemical profiling and molecular analyses. There are known genes, such as the phytoene synthase (PSY1), β-carotene hydroxylase (CHYB), lycopene β, and ξ cyclase (LYCB and LYCE), that play significant roles in the biosynthesis of carotenoids in plants [
115,
116]. Hence, the screening of the cowpea diversity panels targeting these genes can reduce the cost and time needed for profiling and help to precisely identify accessions with the trait of interest.
Genomics interventions for important and quantitative traits such as carotenoid content can begin at the early stage of the breeding scheme by tapping into the genetic and genomics resources of the crop [
11,
110]. The advances in cowpea genomics enabled the development of a reference genome [
117], genetic linkage maps [
118,
119,
120,
121], and diverse molecular markers and marker systems (RFLP, SNPs, SSRs, KAPs, etc.) [
122,
123,
124] to support the development of improved cowpea varieties. The assessment of genetic diversity for carotenoid content in cowpea can, therefore, be conducted along with screening and validation of the existing markers and their possible association with those known genes involved in carotenoid biosynthesis to identify quantitative trait loci amenable to the smooth implementation of marker-assisted selection (MAS) for enhanced carotenoid content in cowpea.
To anticipate the low genetic diversity reported in cowpea [
112], deploying mutagenesis [
125] will help to broaden the diversity in the crop. For this purpose, the use of TILLING (Targeting Induced Local Lesions In Genomes), a technique that combines chemical mutagenesis and high-throughput screening of SNPs by mismatch detection [
126], will help to achieve fast progress in broadening the genetic basis and improving the carotenoid content in cowpea.
Once the working germplasm with the elite or potential genotypes is identified, the next step involves embracing hybridization between accessions. Notably, at this stage, the objective consists of conducting smart combinations among genotypes using appropriate mating design (Diallel, North Carolina Mating Design) and population development techniques (Single Seed descent and Backcross) [
127,
128], which will help to estimate the variance components, gene actions as well as heritability [
127] in order to dissect the genetic architecture of carotenoid biosynthesis in cowpea to support the breeding scheme. Previous research indicated the predominance of additive gene effects over the effects of non-additive genes in the inheritance of carotenoid content in plants [
129,
130], suggesting that the use of the proposed designs can also facilitate introgression of the trait into elite and farmers’ preferred cowpea cultivars.
2. Harnessing the Power of Plant Factory System, Speed Breeding, and Omics
Facing the increasing demand for quantity and quality foods to feed an ever-growing population, there has been a steady shift from traditional rain-fed agriculture to indoor growing systems/vertical farming. The evolution of this approach has given rise to the plant factory system, which is referred to as a closed plant production system in which ventilation is kept at a minimum, and artificial light is used as the sole light source for plant growth [
131]. The adoption of this production practice has been very fast in horticultural crops. Nowadays, vegetables, such as spinach, tomato, and kale, are produced in factory systems [
132,
133,
134], which have been customized to meet specific market demands including high-phytochemical and nutrient-dense products. This system can now be extended to agricultural crops with the recent development of the speed breeding method [
135].
Speed breeding is a customized plant factory system for field crops in fully enclosed, controlled-environment growth chambers, which enables the production of many generations, up to six generations of crops per year [
135]. Speed breeding shortens the growth cycle and the time needed for developing new crop varieties. The technology was first implemented for long-day crops such as wheat and canola [
135] and has recently been extended to short-day crops [
136], suggesting that the system can be optimized for cowpea.
As highlighted earlier, light is one of the factors influencing carotenoid biosynthesis in cowpea. The manipulation of light signaling can help to alter the color and nutritional value in plants, resulting in the production of novel functional foods [
20]. Therefore, the optimization of the speed breeding technology for cowpea can help to control the lighting characteristics (intensity and duration) for increased carotenoid contents in the edible part of the plants, including sprouts, leaves. and green pods, to be used as functional foods. This growth system also offers the flexibility of a choice of plant-growing substrate (e.g., rockwool, top soil) to monitor the nutrition of the plants and to apply appropriate chemical elicitors that have a positive impact on carotenoid biosynthesis in plants [
100,
137].
Furthermore, the implementation of this approach in cowpea can also take advantage of the advances in the field of phenomics to deploy non-destructive tools for carotenoid detection and quantification [
64,
138,
139] in cowpea. This will help to minimize the cost of extensive profiling and also generate quality phenotypic data [
140] to guide understanding of the physiological and genetic basis of carotenoid biosynthesis in cowpea. Notably, the metabolomics regulation network of carotenoid biosynthesis in cowpea is still not fully documented.
3. Genetic Engineering for Increasing Carotenoid Content in Cowpea
Genetic engineering has emerged as a technology to overcome the slow process of conventional breeding and the lack of diversity for carotenoid traits among plant germplasm [
142]. A recent report [
25] on the side effects of domestication on the nutritional quality of legume crops in the Fabaceae family highlighted the decline in the contents of carotenoids following the domestication process. According to these authors, there was a decrease in carotenoid content (0.6 ± 0.1 μg/g) in the cultivated cowpea, about three-fold of the content in the wild cowpea (2.3 ± 0.5 μg/g) due to domestication. The wild cowpea gene pool may, therefore, be a source of favorable genes for increased carotenoid content in cowpea
To date, there is no evidence of genetically modified (GM) biofortified legume grains or pulses [
143]. A comparative study of the mechanisms controlling the biosynthesis in the wild and cultivated cowpea plant will provide an avenue to perform guided mutations in the cultivated cowpea genome. The CRISPR/Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats/Cas9 protein) gene-editing technology can assist in precisely conducting the target mutation [
142,
144,
145]. A similar approach was adopted in sweet potato with an increase of 4- to 130-fold of zeaxanthin content in the transgenic potato [
146]. On the other hand, interspecific genes transfer between the wild cowpea and the cultivated cowpea genome through genetic transformation [
147,
148,
149]. In addition, the donor organisms could be from closely related species in the Fabaceae family and plant or animal species with the genes of interest. For instance, metabolic engineering of the phytoene synthase gene (crtB) from bacteria (
Erwinia uredovora) helped to achieve a 150-fold increase in β-carotene in transgenic eggplant callus [
148]. The duplication of these approaches in cowpea can also help increase the carotenoid content. However, the environmental and health concerns about genetic engineering and its products globally, and especially in many regions where cowpea is a staple food crop, seem to portray genetic engineering as an avenue of last resort. Nonetheless, some tangible progress has been made with the adoption of Bt cowpea in Nigeria [
150], the leading cowpea producer in the world, indicating a promising future to escalate this technique for increasing carotenoids in cowpea. Hence, continuous public awareness raising and increasing advocates in the private and public partnerships to scale up the research technologies in developing countries will be a strong levier in the successful deployment of genetic engineering for micronutrients including carotenoid contents in cowpea.
This entry is adapted from the peer-reviewed paper 10.3390/plants13030412