Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Pharmacology & Pharmacy
Lysozyme, especially the one obtained from hen’s egg white, continues to show new pharmacological properties. Lysozyme can interact with nucleic acids and alter their function, but this effect is uncoupled from the catalytic activity that determines its antibacterial activity; it is present in intact lysozyme but is equally potent in a heat-degraded lysozyme or in a nonapeptide isolated by proteolytic digestion.
- antiviral activity
1. Introduction
Lysozyme is a term used to describe dozens of peptides found in the animal and plant world that share a highly conserved chemical structure, albeit with some, sometimes significant, differences between them [1]. Human lysozyme is, of course, one of the most studied lysozymes [2,3], followed by hen egg white lysozyme (HEWL), the only lysozyme also available in a pharmaceutical form and used for therapeutic purposes (Figure 1) [4,5,6]. Since Sir Alexander Fleming described its biological properties and medical potential [7], it has been present in research laboratories for over 100 years. In 1953, in a scientific meeting at the New York Academy of Medicine, Meyer highlighted the biological properties of this enzyme, pointing out its antibacterial activity and, in particular, its biological and metabolic functions [8]. Although it has not achieved the success we would have expected, e.g., as an antibacterial drug [9,10,11], apart from its use in the food industry [12], it has become one of the most studied molecules in the biological and medical sciences and one of the most widely used models in biochemistry [13,14,15].
2. Lysozyme Modulates Nucleic Acid Activity
Lysozyme is able to interact with nucleic acids, as shown by the results of gel electrophoresis, enzymatic activity, and co-precipitation studies [29], which demonstrate that both HEWL and human lysozyme are able to bind with DNA and RNA. These studies emphasize that DNA requires specific conformations, and that the interaction is physical and electrostatic in nature, as lysozyme is easily separated from a nucleic acid (Figure 3). From a study designed to demonstrate that ATP binds to proteins, modifying their structure and modulating their functions, it appears that lysozyme has multiple binding sites, including one known high-affinity nucleic acid binding site and five non-specific, previously unknown sites [30]. This hypothesis, which is supported by the results of studies by Lee-Huang et al. [31], demonstrating the protective effect of lysozyme on HIV infection, states that lysozyme is actively involved in the processes of viral transcription and replication [29] as an original part of the body’s defenses [32], which indicates that the interaction of lysozyme with DNA molecules can disrupt DNA replication, modulate gene expression, and influence bacterial infections, in addition to HIV viruses.
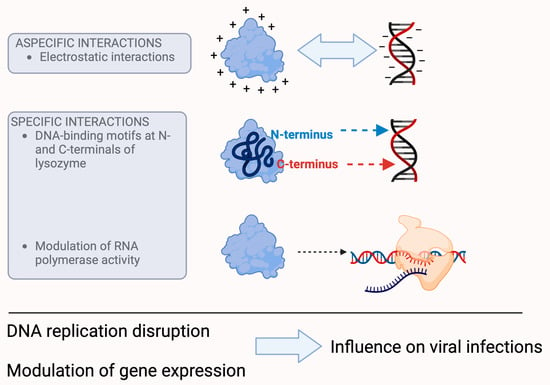
Figure 3. Presumed mechanisms of the antiviral effect of lysozyme. Lysozyme is able to interact with nucleic acids because its positive charge alters the charge and electrophoretic mobility of DNA, which affects transcription, translation, and cell division. The latter may also be the result of specific interactions, as lysozyme has DNA-binding motifs at the N- and C-terminals of its molecule. In addition, lysozyme is able to control the activity of RNA polymerase. All these processes can ultimately lead to the control of viral infectivity. (For further information about the hypotheses of the antiviral effect of lysozyme, please refer to [29,33,34].)
On the other hand, lysozyme, with its strong positive charge (+8e), its compact structure, and depending on its concentration, is able to alter the charge and electrophoretic mobility of DNA [35,36], a fundamental event that plays an important role in cellular processes such as transcription, translation, and cell division. However, these biological properties may also be the result of specific interactions, in addition to electrostatic interactions, which also play an important role in the binding of lysozyme with nucleic acids, as confirmed with surface plasmon resonance spectroscopy studies that identified DNA-binding motifs at the N- and C- terminals of lysozyme [33].
Studies with T7 lysozyme, a lysozyme that exerts its catalytic activity at an amide bond rather than a glycosidic bond [37], have shown that it is able to control the activity of RNA polymerase by inhibiting both the initiation and continuation of its activity [34]. Although this study was performed with a purified material of bacteriophage origin, the signal is evident in its ability to modulate RNA synthesis, and this effect can have devastating consequences for viruses, especially those that rely on RNA. The inhibition of RNA polymerase leads to the inhibition of transcription [38,39], which ultimately leads to the control of infectivity [40].
The possibility that lysozyme can bind nucleic acids arises from studies that have demonstrated the similarity of this peptide to histones [29]. The recent study by Liu et al. [41] builds on these observations and uses PCR to investigate the effects of lysozyme on the replication and transcription of nucleic acids and finds that the inhibitory effect caused by lysozyme is strong and mediated by the interaction of lysozyme with RNA polymerase, and it concludes that this activity could explain the observed antiviral effect. The fact that lysozyme is able to bind peptides is nothing new. For example, two lysozymes from hen and turkey egg whites were analyzed using X-ray diffraction and were found capable of binding with the antibody fragment 1F9 [42]. If the crystalline structure is compared with a model of the single-chain Fv fragment 1F9 complexed with HEWL, it can be seen that there is a collision between Asp101 in the lysozyme and Trp98 of the complementarity-determining region H3 of the variable domain of the heavy chain.
3. Chemical Structure and Anti-Viral Activity
Studies on the interactions of lysozyme with nucleic acids suggest that lysozyme is able to influence DNA replication by interacting directly with it, but it is also able to modulate gene expression by interacting with RNA polymerase. The obvious question to ask now is what role does the chemical structure of lysozyme play in its antiviral activity? The antiviral activity of lysozyme is attributed to several lysozymes, including HEWL and human lysozyme (see the review [44]), and is attributed to a mechanism of action independent of muramidase activity, which is related to the positive charge of the molecule [45]. The indirect evidence for the antiviral activity of lysozyme comes from a study showing the presence of this peptide in the preparations of human chorionic gonadotropin [31]. One of the isolated peptides has a sequence and muramidase activity comparable to those of human lysozyme and HEWL and showed an antiviral activity without a cytotoxic effect on eukaryotic cells in tests with mononuclear cells exposed to the HIV virus type 1, as shown by the study of 3H-thymidine incorporation. However, the mechanism of the antiviral activity was not clarified in this study.
An attempt to modify the chemical structure of lysozyme to achieve antiviral activity was made by Oeverman et al. [46], who introduced a hydrophobic and negatively charged function (3-hydroxyphthalic anhydride) into the lysozyme molecule from hen egg white. The modified lysozyme molecule showed a remarkable in vitro antiviral activity in a herpes simplex virus model, both in terms of preventing cell infection and, most importantly, and inhibiting virus replication when the drug was added to cells previously infected with the virus (the IC50 decreased from 170 mg/mL to 6 mg/mL). The same study also showed that the proteolytic digestion of lysozyme with trypsin, chymotrypsin, and pepsin produces fragments with no or significantly lower antiviral activity, often only when used as a mixture, and almost always accompanied by some cytotoxicity to the eukaryotic cells.
However, a very detailed study on the antiviral activity of lysozyme fragments obtained using proteolytic digestion with clostripain, which cleaves at the level of arginine residues in the C-terminal region, contains very interesting data [47]. This study highlights a nonapeptide, with the sequence RAWVAWRNR, which corresponds to residues 107–115 of the human lysozyme and appears to be the smallest lysozyme fragment capable of exerting antiviral activity (the prevention of infection and the inhibition of HIV viral replication) comparable to that of the intact lysozyme. Interestingly, this nonapeptide, which is present in the native lysozyme as an α-helix, belongs to a part of the lysozyme that is distinctly different from the catalytic site that exerts muramidase activity (Figure 4). It was later shown that this nonapeptide interacts in nanomolar concentrations with an HIV envelope glycoprotein that is critical for viral entry into cells. The study highlighted the nonapeptide component (two Trp residues separated by two others) that allows it to interact with the hydrophobic pocket of the gp41 glycoprotein and inhibit its fusion with the membrane of the cell to be infected [48]. An investigation of the mechanism of the antiviral effect showed that the nonapeptide modulates gene expression in HIV-infected cells, particularly of genes involved in survival and stress signaling. Similar effects were also demonstrated by transcriptomic analysis in eukaryotic cells exposed to intact HEWL [49]. This study documented the robust evidence on the modulation of genes associated with anti-inflammatory effects with relatively low doses (micromolar range) and short times of cell exposure (1 h).
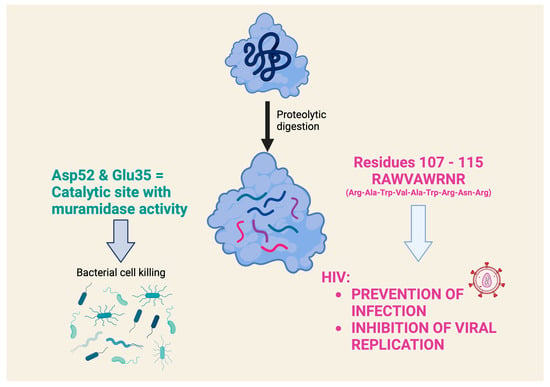
Figure 4. What role does the chemical structure of lysozyme play in antiviral activity? The antiviral activity of several lysozymes is attributed to a mechanism of action independent of muramidase activity, which produces the lytic antibacterial activity. A nonapeptide (RAWVAWRNR), which corresponds to residues 107–115 of the human lysozyme, appears to be the smallest lysozyme fragment that can exert an antiviral effect. This nonapeptide belongs to a part of the lysozyme that is clearly distinct from the catalytic site that exerts muramidase activity [45,47].
Apart from the discussion on the part of the lysozyme molecule responsible for the antiviral activity, it is interesting to mention the activity of the recombinant CCR5 protein into which lysozyme has been incorporated. The transformed chemokine is able to exert a biphasic effect on HIV infection in a number of eukaryotic cell models: it weakly promotes infection at low doses (10−10–10−12 M) and markedly inhibits infection at high doses (10−6–10−9 M). The mechanism appears to be related to the downregulation of the wild-type CCR5 in eukaryotic cells [50].
4. Antiviral Activity: Disinfection over Norovirus Food Contamination
The first evidence for the effect of lysozyme on noroviruses comes from a paper published in 2015 by Takahashi et al., a research group that investigated this effect in detail [51]. Noroviruses usually infect humans through the consumption of food, often vegetables, although not exclusively, in the raw state or through direct contact with infected persons or their fluids, leading to gastroenteritis [52]. The work of Takahashi et al. emphasizes two things: the ability of lysozyme to eliminate contamination of food by the virus, and the fact that this activity comes from a component of lysozyme obtained after heat denaturation of the peptide. Using the murine norovirus model MNV-1, the mechanism of the antiviral effect of lysozyme subjected to heat denaturation was demonstrated. A classical heat denaturation of lysozyme was performed by dissolving lysozyme from hen egg white in 5% distilled water. The solution was filtered through a 0.20 μm filter, and the filtrate was heated to 100 °C in a bath for 40 min then cooled on ice for final preparation of the heat-denatured lysozyme.
Heat denaturation of lysozyme produces a chemical species that has completely lost its enzymatic activity but has increased antibacterial activity, including many Gram-negative bacteria. This activity is explained by the dimerization of the molecule and the exposure of an active site rich in tryptophan groups that allow interaction with the bacterial membrane, making the phospholipid membrane permeable and causing bacterial death [53]. It has also been suggested that the heat-denatured lysozyme is active against herpes simplex viruses in vitro, as its basic nature favors interaction with the viral particles [45,54]. The exposure of hydrophobic amino acids (residues 5–39, according to [51]) could also explain the antiviral activity. Indeed, these residues can interact with the structural proteins of the MNV1 virus, which contain many hydrophobic amino acids, and this interaction leads to inactivation of the virus [55]. However, there is no clear study in the literature to describe in detail the mechanism of action of the heat-denatured lysozyme in destroying capsid proteins and contributing to virus death.
The same research group had already demonstrated in detail, also using the murine norovirus MNS-1 model, the ability of the heat-denatured lysozyme to counteract contamination of fresh vegetables such as salads and their dressings, whereby the infectivity measured as PFU (Plaque Forming Units) was reduced by 2.6 or 4 logs compared to the untreated control [56]. Always using the MNV-1 mouse norovirus model, the containment of food infection by lysozyme was also demonstrated in some bread fillings, where treatment with the heat-denatured lysozyme immediately after MNV-1 inoculation completely eliminated viral contamination [57].
Heat-inactivated lysozyme has also been found to inactivate single-stranded RNA viruses that can cause hepatitis A (HAV). It is known that various foods, from seafood to strawberries, can be contaminated with HAV and cause hepatitis which, although not comparable to hepatitis B and C, can be very disabling [58,59]. Also in this case, a laboratory simulation with three HAV types with three different genotypes treated with a 1% solution of the heat-denatured lysozyme for 60 min showed a 2.6 log reduction in viral contamination compared to the control [60]. (For a general overview on use of lysozyme as a food preservative see Figure 5).
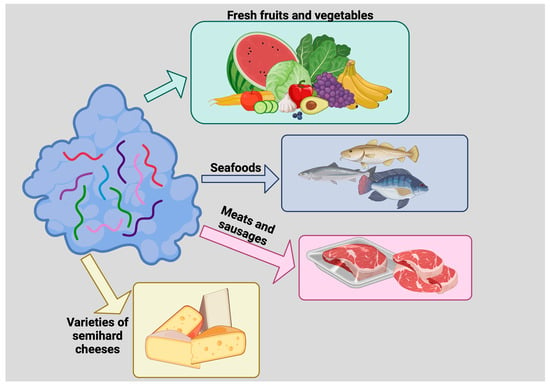
Figure 5. Use of lysozyme as a food preservative. Lysozyme inhibits the growth of deleterious microorganisms, thus prolonging food shelf life. Lysozyme has been used to preserve fresh fruits and vegetables, seafoods, meats and sausages, and varieties of semihard cheeses such as Edam, Gouda, and some Italian cheeses.
This entry is adapted from the peer-reviewed paper 10.3390/molecules29030652
This entry is offline, you can click here to edit this entry!
