Breast cancer accounted for 685,000 deaths globally in 2020, and half of all cases occur in women with no specific risk factor besides gender and age group. The number of mastectomies performed for younger women increased, raising the need for adequate breast reconstructive surgery. Advances in oncological treatment have made it possible to limit the extent of what represents radical surgery for breast cancer, a marked trend toward mastectomies in breast-conserving surgery-eligible patients are seen. Prophylactic mastectomies have also registered an upward trend. This trend together with new uses for breast reconstruction like chest feminization in transgender patients has increased the need for breast reconstruction surgery
- breast reconstruction
- reconstruction following mastectomy
- prophylactic mastectomy
- chest feminization
- transgender
- implant reconstruction of breast
- immediate reconstruction
- delayed reconstruction
- two-stage breast reconstruction
1. Introduction
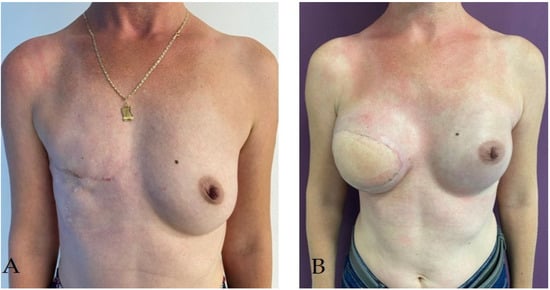
2. Breast Reconstruction
2.1. Timing of Breast Reconstruction—Immediate or Delayed
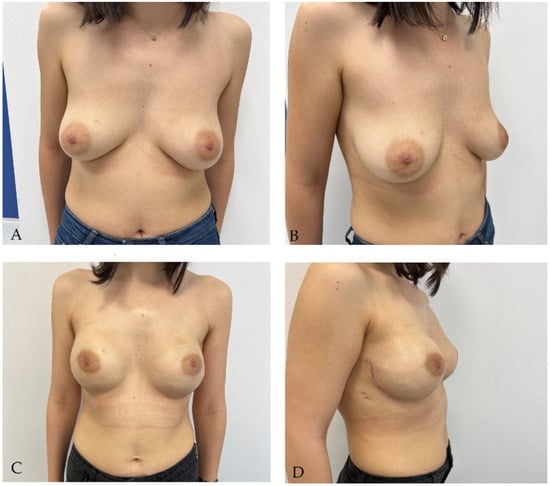
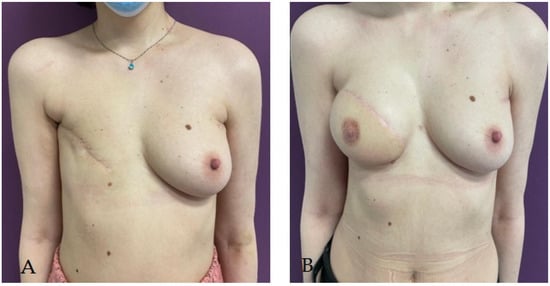
2.2. Two-Stage Breast Reconstruction
2.3. Breast Reconstruction with Implant
2.4. Autologous Breast Reconstruction Techniques
-
The advantages of flap reconstruction.
- b.
-
Types of transferred free flaps.
- c.
-
Latissimus dorsi pediculated flap.
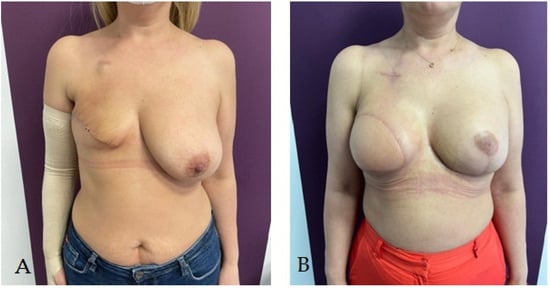
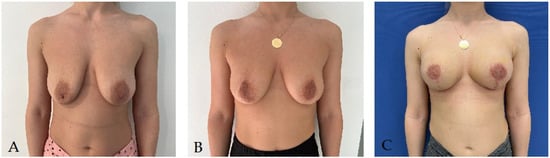
This entry is adapted from the peer-reviewed paper 10.3390/life14010138
References
- WHO. Statistics on Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 22 July 2023).
- Kummerow, K.L.; Du, L.; Penson, D.F.; Shyr, Y.; Hooks, M.A. Nationwide Trends in Mastectomy for Early-Stage Breast Cancer. JAMA Surg. 2015, 150, 9–16.
- Tuttle, T.M.; Habermann, E.B.; Grund, E.H.; Morris, T.J.; Virnig, B.A. Increasing Use of Contralateral Prophylactic Mastectomy for Breast Cancer Patients: A Trend toward More Aggressive Surgical Treatment. J. Clin. Oncol. 2007, 25, 5203–5209.
- Soran, A.; Kamali Polat, A.; Johnson, R.; McGuire, K.P. Increasing Trend of Contralateral Prophylactic Mastectomy: What Are the Factors behind This Phenomenon? Surgeon 2014, 12, 316–322.
- Patel, H.; Samaha, Y.; Ives, G.; Lee, T.-Y.; Cui, X.; Ray, E. Chest Feminization in Male-to-Female Transgender Patients: A Review of Options. Transgender Health 2021, 6, 244–255.
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer Incidence and Mortality Patterns in Europe: Estimates for 40 Countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403.
- Sancho-Garnier, H.; Colonna, M. Epidémiologie Des Cancers Du Sein Breast Cancer Epidemiology. Presse Medicale 2019, 48, 1076–1084.
- Simion, L.; Rotaru, V.; Cirimbei, C.; Gales, L.; Stefan, D.-C.; Ionescu, S.-O.; Luca, D.; Doran, H.; Chitoran, E. Inequities in Screening and HPV Vaccination Programs and Their Impact on Cervical Cancer Statistics in Romania. Diagnostics 2023, 13, 2776.
- Simion, L.; Chitoran, E.; Cirimbei, C.; Stefan, D.-C.; Neicu, A.; Tanase, B.; Ionescu, S.O.; Luca, D.C.; Gales, L.; Gheorghe, A.S.; et al. A Decade of Therapeutic Challenges in Synchronous Gynecological Cancers from the Bucharest Oncological Institute. Diagnostics 2023, 13, 2069.
- UpToDate. Overview of Breast Reconstruction. Available online: https://www.uptodate.com/contents/overview-of-breast-reconstruction?search=Overview%20of%20breast%20reconstruction&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1 (accessed on 21 July 2023).
- Angelos, P.; Bedrosian, I.; Euhus, D.M.; Herrmann, V.M.; Katz, S.J.; Pusic, A. Prophylactic Mastectomy: Challenging Considerations for the Surgeon. Ann. Surg. Oncol. 2015, 22, 3208–3212.
- Dragun, A.E.; Pan, J.; Riley, E.C.; Kruse, B.; Wilson, M.R.; Rai, S.; Jain, D. Increasing Use of Elective Mastectomy and Contralateral Prophylactic Surgery among Breast Conservation Candidates: A 14-Year Report from a Comprehensive Cancer Center. Am. J. Clin. Oncol. 2013, 36, 375–380.
- Metcalfe, K.A.; Eisen, A.; Poll, A.; Candib, A.; McCready, D.; Cil, T.; Wright, F.; Demsky, R.; Mancuso, T.; Sun, P.; et al. Frequency of Contralateral Prophylactic Mastectomy in Breast Cancer Patients with a Negative BRCA1 and BRCA2 Rapid Genetic Test Result. Ann. Surg. Oncol. 2021, 28, 4967–4973.
- Mathelin, C.; Bruant-Rodier, C. Indications for Breast Reconstruction after Mastectomy According to the Oncological Situation. Ann. Chir. Plast. Esthet. 2018, 63, 580–584.
- Atisha, D.; Alderman, A.K.; Lowery, J.C.; Kuhn, L.E.; Davis, J.; Wilkins, E.G. Prospective Analysis of Long-Term Psychosocial Outcomes in Breast Reconstruction: Two-Year Postoperative Results from the Michigan Breast Reconstruction Outcomes Study. Ann. Surg. 2008, 247, 1019–1028.
- Platt, J.; Zhong, T. Patient-Centered Breast Reconstruction Based on Health-Related Quality-of-Life Evidence. Clin. Plast. Surg. 2018, 45, 137–143.
- Zhong, T.; McCarthy, C.; Min, S.; Zhang, J.; Beber, B.; Pusic, A.L.; Hofer, S.O.P. Patient Satisfaction and Health-Related Quality of Life after Autologous Tissue Breast Reconstruction: A Prospective Analysis of Early Postoperative Outcomes. Cancer 2012, 118, 1701–1709.
- Zhong, T.; Hu, J.; Bagher, S.; Vo, A.; O’Neill, A.C.; Butler, K.; Novak, C.B.; Hofer, S.O.P.; Metcalfe, K.A. A Comparison of Psychological Response, Body Image, Sexuality, and Quality of Life between Immediate and Delayed Autologous Tissue Breast Reconstruction: A Prospective Long-Term Outcome Study. Plast. Reconstr. Surg. 2016, 138, 772–780.
- Simmons, R.M.; Fish, S.K.; Gayle, L.; La Trenta, G.S.; Swistel, A.; Christos, P.; Osborne, M.P. Local and Distant Recurrence Rates in Skin-Sparing Mastectomies Compared With Non-Skin-Sparing Mastectomies. Ann. Surg. Oncol. 1999, 6, 676–681.
- Toth, B.A.; Lappert, P. Modified Skin Incisions for Mastectomy: The Need for Plastic Surgical Input in Preoperative Planning. Plast. Reconstr. Surg. 1991, 87, 1048–1053.
- Kroll, S.; Khoo, A.; Singletary, E.; Ames, F.; Wang, B.G.; Reece, G.; Miller, M.; Evans, G.; Robb, G. Local Recurrence Risk after Skin-Sparing and Conventional Mastectomy: A 6-Year Follow-Up. Plast. Reconstr. Surg. 1999, 104, 421–425.
- Galimberti, V.; Vicini, E.; Corso, G.; Morigi, C.; Fontana, S.; Sacchini, V.; Veronesi, P. Nipple-Sparing and Skin-Sparing Mastectomy: Review of Aims, Oncological Safety and Contraindications. Breast 2017, 34, S82–S84.
- Radu, M.; Bordea, C.; Noditi, A.; Blidaru, A. Assessment of Mastectomy Skin Flaps for Immediate Implant-Based Breast Reconstruction. J. Med. Life 2018, 11, 137–145.
- Huang, C.J.; Hou, M.F.; Lin, S.D.; Chuang, H.Y.; Huang, M.Y.; Fu, O.Y.; Lian, S.L. Comparison of Local Recurrence and Distant Metastases between Breast Cancer Patients after Postmastectomy Radiotherapy with and without Immediate TRAM Flap Reconstruction. Plast. Reconstr. Surg. 2006, 118, 1079–1086.
- Gieni, M.; Avram, R.; Dickson, L.; Farrokhyar, F.; Lovrics, P.; Faidi, S.; Sne, N. Local Breast Cancer Recurrence after Mastectomy and Immediate Breast Reconstruction for Invasive Cancer: A Meta-Analysis. Breast 2012, 21, 230–236.
- Strålman, K.; Mollerup, C.L.; Kristoffersen, U.S.; Elberg, J.J. Long-Term Outcome after Mastectomy with Immediate Breast Reconstruction. Acta Oncol. 2008, 47, 704–708.
- Kronowitz, S.J. Delayed-Immediate Breast Reconstruction: Technical and Timing Considerations. Plast. Reconstr. Surg. 2010, 125, 463–474.
- Gradishar, W.; Salerno, K.E. NCCN guidelines update: Breast cancer. J. Natl. Compr. Cancer Netw. 2016, 14, 641–644.
- Harris, E.E.R.; Freilich, J.; Lin, H.Y.; Chuong, M.; Acs, G. The Impact of the Size of Nodal Metastases on Recurrence Risk in Breast Cancer Patients with 1-3 Positive Axillary Nodes after Mastectomy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 609–614.
- McBride, A.; Allen, P.; Woodward, W.; Kim, M.; Kuerer, H.M.; Drinka, E.K.; Sahin, A.; Strom, E.A.; Buzdar, A.; Valero, V.; et al. Locoregional Recurrence Risk for Patients with T1,2 Breast Cancer with 1-3 Positive Lymph Nodes Treated with Mastectomy and Systemic Treatment. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 392–398.
- Kronowitz, S.J.; Hunt, K.K.; Kuerer, H.M.; Babiera, G.; McNeese, M.D.; Buchholz, T.A.; Strom, E.A.; Robb, G.L. Delayed-Immediate Breast Reconstruction. Plast. Reconstr. Surg. 2004, 113, 1617–1628.
- Doherty, C.; Mcclure, J.A.; Baxter, N.N.; Brackstone, M. Complications from Postmastectomy Radiation Therapy in Patients Undergoing Immediate Breast Reconstruction: A Population-Based Study. Adv. Radiat. Oncol. 2023, 8, 101104.
- See, M.S.F.; Farhadi, J. Radiation Therapy and Immediate Breast Reconstruction: Novel Approaches and Evidence Base for Radiation Effects on the Reconstructed Breast. Clin. Plast. Surg. 2018, 45, 13–24.
- Bellini, E.; Pesce, M.; Santi, P.L.; Raposio, E. Two-Stage Tissue-Expander Breast Reconstruction: A Focus on the Surgical Technique. Biomed. Res. Int. 2017, 2017, 1791546.
- Cemal, Y.; Albornoz, C.R.; Disa, J.J.; Mccarthy, C.M.; Mehrara, B.J.; Pusic, A.L.; Cordeiro, P.G.; Matros, E. A Paradigm Shift in U.S. Breast Reconstruction: Part 2. the Influence of Changing Mastectomy Patterns on Reconstructive Rate and Method. Plast. Reconstr. Surg. 2013, 131, 320e–326e.
- Sherine, E.G.; Woods, J.E.; O’Fallon, M.; Beard, M.; Kurland, L.T.; Melton, J.L. Complications Leading to Surgery after Breast Implantation. N. Engl. J. Med. 1997, 336, 677–682.
- Percec, I.; Bucky, L.P. Successful Prosthetic Breast Reconstruction after Radiation Therapy. Ann. Plast. Surg. 2008, 60, 527–531.
- Thamm, O.C.; Andree, C. Immediate Versus Delayed Breast Reconstruction: Evolving Concepts and Evidence Base. Clin. Plast. Surg. 2018, 45, 119–127.
- Nahabedian, M.Y. Acellular Dermal Matrices in Primary Breast Reconstruction. Plast. Reconstr. Surg. 2012, 130, 44S–53S.
- Uitto, J.; Olsen, D.R.; Fazio, M.J. Extracellular Matrix of the Skin: 50 Years of Progress. J. Investig. Dermatol. 1989, 92, S61–S77.
- Ho, A.Y.; Hu, Z.I.; Mehrara, B.J.; Wilkins, E.G. Radiotherapy in the Setting of Breast Reconstruction: Types, Techniques, and Timing. Lancet Oncol. 2017, 18, e742–e753.
- Chen, V.W.; Lin, A.; Hoang, D.; Carey, J. Trends in Breast Reconstruction Techniques at a Large Safety Net Hospital: A 10-Year Institutional Review. Ann. Breast Surg. 2018, 2, 14.
- Koshima, I.; Soeda, S. Inferior Epigastric Artery Skin Flaps without Rectus Abdominis Muscle. J. Plast. Surg. 1989, 42, 645–648.
- Healy, C.; Allen, R.J. The Evolution of Perforator Flap Breast Reconstruction: Twenty Years after the First DIEP Flap. J. Reconstr. Microsurg. 2014, 30, 121–126.
- Maxwell, P. Iginio Tansini and the Origin of the Latissimus Dorsi musculocutaneous Flap. Plast. Reconstr. Surg. 1989, 65, 686–692.
- Champaneria, M.C.; Wong, W.W.; Hill, M.E.; Gupta, S.C. The Evolution of Breast Reconstruction: A Historical Perspective. World J. Surg. 2012, 36, 730–742.
- Bostwick, J.; Vasconez, L.O.; Jurkiewicz, M.J. Breast Reconstruction after a Radical Mastectomy. Plast. Reconstr. Surg. 1978, 61, 682–693.
- Schneider, W.J.; Hill, H.L.; Brown, R.G. Latissimus dorsi myocutaneous flap for breast reconstruction. Br. J. Plast. Surg. 1977, 30, 277–281.
- Papp, C.; McCraw, J.B. Autogenous Latissimus Breast Reconstruction. Clin. Plast. Surg. 1998, 25, 261–266.
- DeLong, M.R.; Tandon, V.J.; Rudkin, G.H.; Da Lio, A.L. Latissimus Dorsi Flap Breast Reconstruction—A Nationwide Inpatient Sample Review. Ann. Plast. Surg. 2017, 78, S185–S188.
- Spear, S.L.; Clemens, M.W. Latissimus Dorsi Flap Breast Reconstruction. In Plastic Surgery, 3rd ed.; Saunders (Elsevier): Philadelphia, PA, USA, 2012; pp. 370–392.
- Hardwicke, J.T.; Prinsloo, D.J. An Analysis of 277 Consecutive Latissimus Dorsi Breast Reconstructions: A Focus on Capsular Contracture. Plast. Reconstr. Surg. 2011, 128, 63–70.
