Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The tick-borne encephalitis virus (TBEV) is the arboviral etiological agent of tick-borne encephalitis (TBE), considered to be one of the most important tick-borne viral diseases in Europe and Asia.
- tick-borne encephalitis
- TBEV
- arbovirus
- Flaviviridae
- Ixodes
- vector-borne
1. Introduction
Tick-borne encephalitis (TBE) is an arboviral disease caused by the TBE virus (TBEV), a member of the Flaviviridae family, as are the etiological agents of dengue fever, yellow fever, and Japanese encephalitis. Tick-borne encephalitis is a serious health problem in Europe and Asia [1][2]. In recent years, a rise in the incidence of TBE as well as an expansion of the geographical range of the disease have been evident. In 2020, 24 European Union/European Economic Area (EU/EEA) member countries reported 3817 cases of TBE [3]. The virus is transmitted between ticks, animals, and humans, so it can be considered in the context of a one health perspective [4]. Humans are incidental and dead-end hosts infected mainly through the bites of hard ticks. Another route of TBEV transmission may be the ingestion of unpasteurized milk or dairy products from infected animals [5].
2. Virus Structure
The tick-borne encephalitis virus is spherical or quasi-spherical, lipid-enveloped, and approximately 50 nm in diameter, and it contains a positive, single-stranded RNA genome that acts as mRNA for translation. Although the approximate size and shape of TBEV and other flaviviruses are estimated, there are variations in size due to factors such as the genetic diversity in the virus population, changes during the maturation process, and the methods used for the imaging and analysis of virions [6]. The virion is composed of three structural proteins: the envelope (E), membrane (M), and capsid (C) proteins (Figure 1). Seven non-structural (NS) proteins—NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5—have also been identified in infected cells. Non-structural proteins play an important role in virus replication, the processing of the structural proteins, and the modulation of host cell function. The M glycoprotein is primarily synthesized as a precursor (prM) that interacts with glycoprotein E and protects its fusion loop from premature activation [7][8]. The nucleocapsid consists of the genome and the C protein and is surrounded by the viral envelope, which consists of both M and E glycoproteins and host-cell-derived lipids. Glycoprotein E is the major antigen of TBEV and is responsible for receptor binding and membrane fusion [8][9]. The glycoprotein-E-coding gene is commonly sequenced and analyzed, but a pairwise distance analysis indicated that it has evolutionary patterns distinct from other TBEV genomic regions [10].

Figure 1. Schematic structure of the TBEV virion, the viral genome (RNA) and the C protein form a nucleocapsid surrounded by a lipid envelope in which glycoproteins E and M are embedded.
In recent years, it has been confirmed that the structure and sequence of non-coding RNA regions of Flavivirus genomes are of great functional importance. The 5′ untranslated region (UTR) and 3′ UTR of TBEV are postulated to be important for genome replication. These noncoding fragments dimerize, leading to the cyclization of the genome via the formation of a panhandle structure [11][12][13]. Hirano et al. reported that a cis-acting RNA element was identified in the 5′ UTR of TBEV that mediates neurovirulence by hijacking the host mRNA transport system, thus allowing the transport of TBEV genomic RNA to neuronal dendrites, where it replicates locally. Neuronal granules are involved in the transport of TBEV genomic RNA, and the hijacking of their system for the transport of viral genomic RNA in dendrites demonstrated in the results of Hirano et al. indicates the neuropathogenicity of the virus and explains how the viral infection can result in severe neurological diseases [14]. A recent study investigated the role of the predicted secondary RNA elements of the first 107 nucleotides of the genome (stem-loop A, SLA) [11]. Mutations within individual SLA structures (Core 0, Stem 1, Stem 2) affected virus replication, infectivity, and spread, but an effect on viral translation was not suggested.
3. Phylogenetic Analysis of Circulating Virus Subtypes
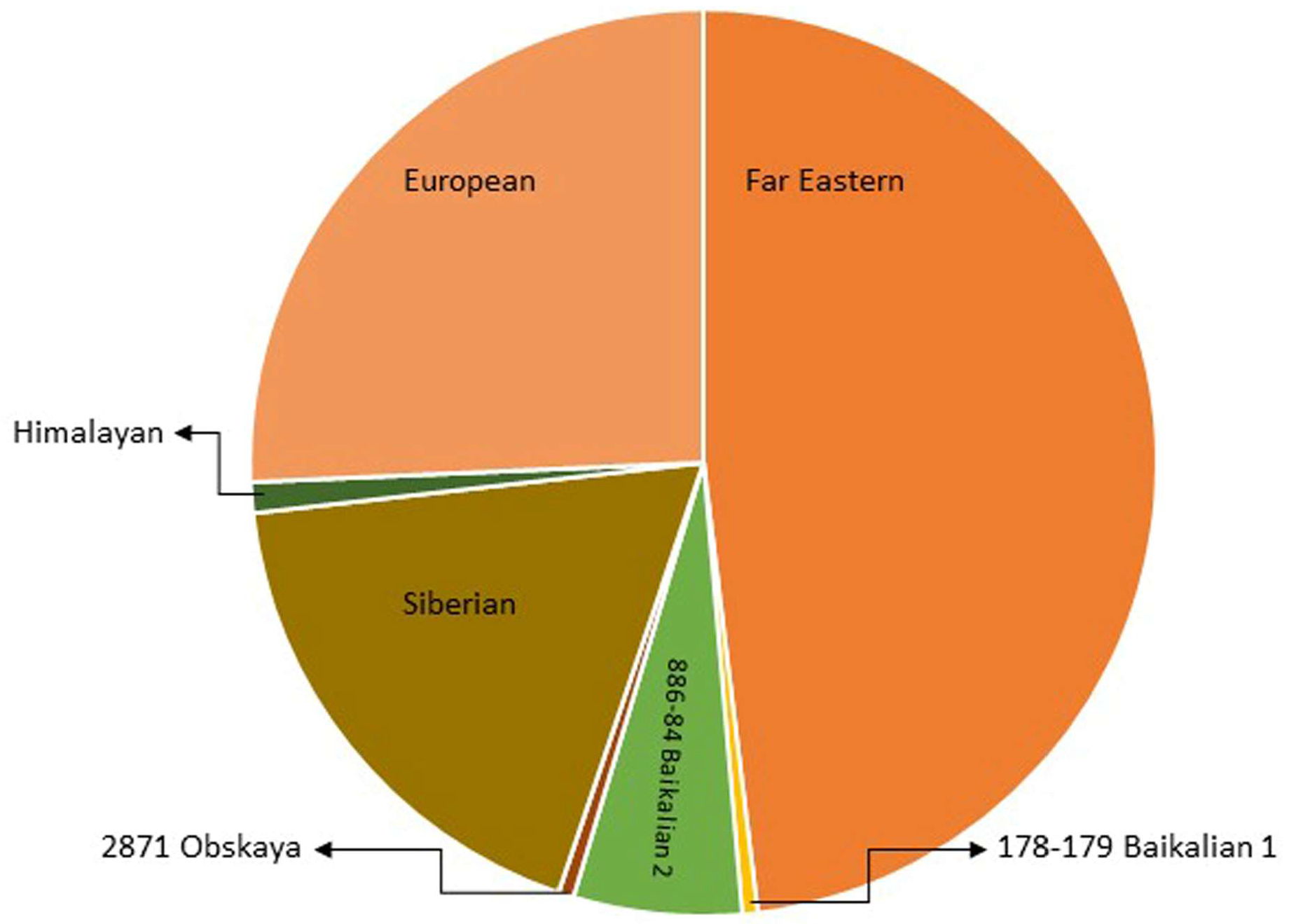
Traditionally, TBEVs have been classified into three subtypes: the European (TBEV-Eu), the Siberian (TBEV-Sib), and the Far-Eastern (TBEV-FE). Recently, Baikalian (TBEV-Bkl) and Himalayan (TBEV-Him) subtypes have been distinguished [15][16][17]. The assumption that if the open reading frame nucleotide sequence of two viruses differs by less than 10%, the two viruses belong to the same subtype guided the recent analyses, and they pointed to seven subtypes of TBEV: TBEV-Eu, TBEV-Sib, TBEV-FE, TBEV-Ob (TBEV-2871), TBEV-Him, TBEV-Bkl-1 (178-79), and TBEV-Bkl-2 (886-84) (Figure 2) [10]. Another phylogenetic analysis using the Nextstrain framework and based on more than 220 complete TBEV genomes proposed TBEV-Bkl1, TBEV-Bkl-2, and TBEV-Him as separate clades in addition to the three major subtypes TBEV-Eu, TBEV-Sib, and TBEV-FE [18].
The viral strains within the three main subtypes of TBEV, TBEV-Eu, TBEV-Sib, and TBEV-FE are believed to be descended from a common ancestor and have evolved independently. Recent studies on TBEV strains isolated near Lake Baikal in Russia, TBEV-Bkl-2 (886-84), have shown that these strains have a mosaic genome: some parts are more closely related to viruses from the Siberian group, while others are more closely related to the Far-Eastern group. Therefore, the Baikalian subtype of TBEV is postulated as evident of recombination between the Siberian and Far-Eastern subtypes [19].
The phylogenetic groups of TBEV may differ in their clinical presentation. For the European subtype, the fatality rate has been estimated at below 2% [20]. The disease caused by European subtypes of TBEV is usually biphasic with a viremic phase associated with a fever and myalgia, and in some patients, is followed by neurological symptoms of varying severity [1]. The Far-Eastern subtype is considered the most pathogenic, with a mortality rate estimated at up to 40% by some authors [21][22]. The Siberian subtype typically results in a less severe disease than that caused by the TBEV-FE subtype, with a fatality rate of 6–8%, and it may be associated with chronic TBE [23]. The TBEV-Ob (2871) strain was isolated from Ixodes pavlovskyi, and it has not been detected in humans, so its pathogenicity to humans is unknown. The virulence of the strain has been confirmed in laboratory mice. According to the classification based on the invasiveness index, the TBEV-Ob strain belongs to the group of the most common strains from Western Siberia [24]. Him-TBEV was detected in a wild rodent, Marmota himalayana, at the Qinghai–Tibet Plateau in China. An analysis of 17 amino acid residues associated with the pathogenicity of TBEV showed that Him-TBEV shares nine substitutions that are specific to pathogenic strains and five substitutions that are specific to strains isolated from subclinical cases. The pathogenic-associated amino acid substitution profile of the Him-TBEV strain is similar to the low-pathogenic TBEV Oshima strain [16]. The ability of TBEV-Bkl-2 (886-84) to cause lethal focal forms of encephalitis, as well as the results of laboratory tests, indicate the high pathogenic potential of this group [25]. However, assigning a certain level of pathogenicity to strains of a given subtype may be misleading. Some studies have shown that different strains within certain TBEV subtypes may show a variable virulence [26][27].
4. TBEV Reservoirs, Vectors, and Transmission
Hard ticks of the family Ixodidae act both as vectors and reservoirs of TBEV. Ixodes ricinus occurs especially in central, northern, and eastern Europe, and I. persulcatus is found in parts of the Baltic States, Finland, Russia, and Siberia [28]. Field studies and experimental findings indicate that other species of ticks might also be effective TBEV vectors. Natural infections with TBEV were reported in 16 species of ixodid ticks more than 30 years ago [6]. Currently, at least eight species are known to be able to transmit the TBE virus, and so far, the virus has been isolated from at least 14 other species [29]. In Central Europe, TBEV has been revealed in some species of “hard” ticks: Ixodes persulcatus, Ixodes ricinus, Ixodes hexagonus [30], Ixodes arboricola [31], Haemaphysalis punctate [32], Haemaphysalis concinna [33], Dermacentor marginatus, and Dermacentor reticulatus [34]. Ixodes gibbosus is considered a marginal vector in the Mediterranean region [32]. Nosek et al. experimentally proved the vector competence of Haemaphysalis inermis for TBEV [35].
TBEV circulates in small, geographically defined areas, so-called “natural foci”. This cycle involves ticks as vectors and small rodents, insectivores, birds, and large mammals as hosts (Figure 3). Ticks can become infected by feeding on viremic hosts (viremic transmission) or by co-feeding with an infected tick (non-viremic transmission) [36]. Other ways of transmitting the virus include vertical transovarial transmission (via eggs laid by an infected female) and transstadial transmission (between developmental stages of ticks). Horizontal sexual transmission may take place between both ticks and warm-blooded hosts [37]. The transstadial and transovarial transmission of TBEV co-exist and take place simultaneously along with sexual, non-viremic, and other transmission modes.
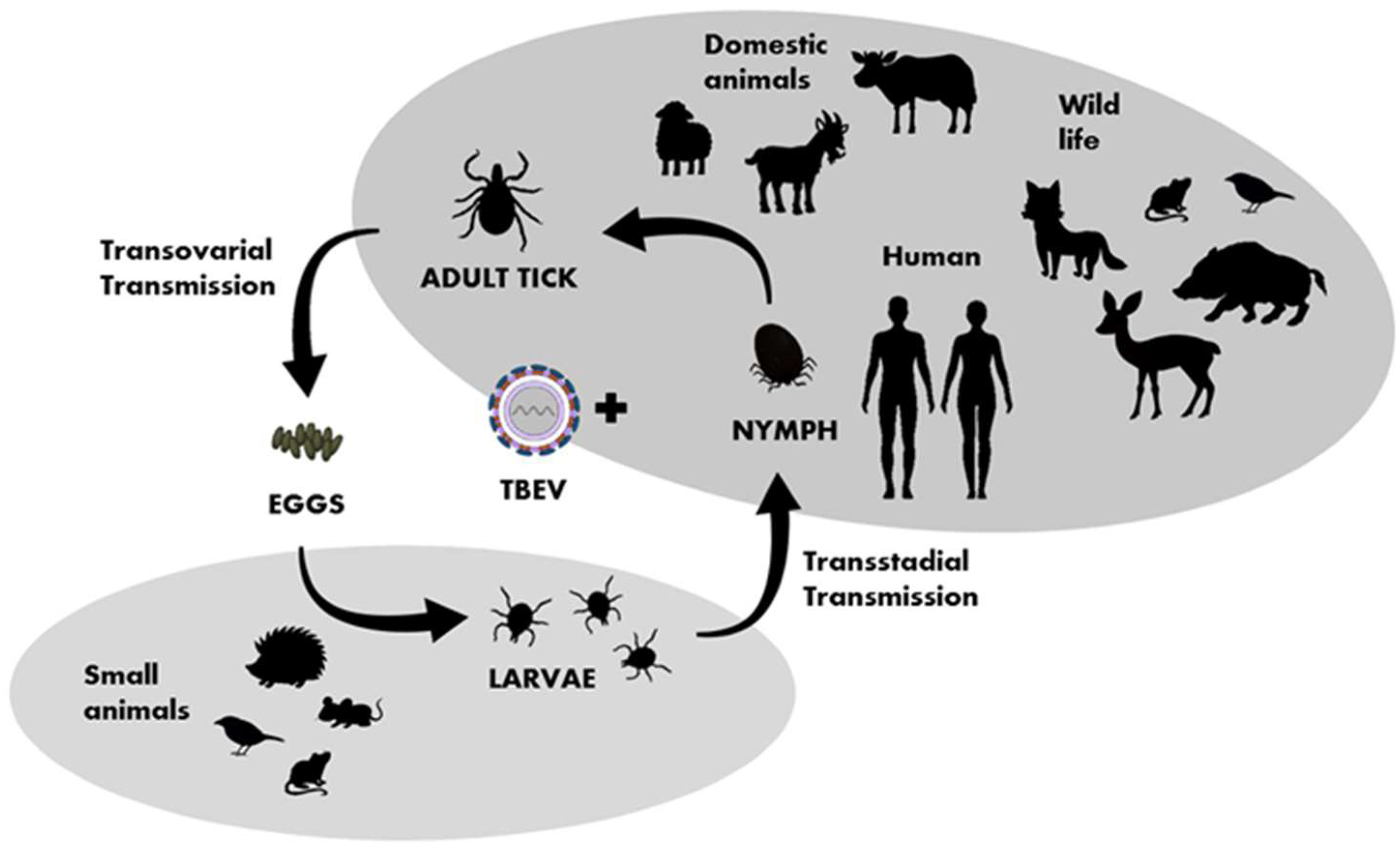
Figure 3. Transmission of TBEV in the life cycle of ixodid ticks, shaded fields indicate the host group characteristic for particular developmental stages of ticks.
The important hosts and reservoirs are small mammals such as rodents (mice and voles), insectivores (hedgehogs and moles), and carnivores (foxes). Rodents are amplifying, asymptomatic hosts of the virus. Prolonged viremia has been confirmed in some rodent species, such as the bank vole (Clethrionomys glareolus). Thus, ticks and small mammals can play a key role in maintaining TBEV in an environment. It is suspected that birds also play a role in the virus’ spread [38][39]. A phylogenetic analysis after the introduction of TBEV to the United Kingdom [40] and studies conducted in Finland and Japan [41][42] indicate the involvement of migrating birds in the spread of the virus. Ticks are believed to have different specific ranges of target animals at different life stages, e.g., adult ticks attack mainly large animals, while nymphs and larvae attach to small- and medium-sized animals, including birds [43][44].
Single cases of TBE transmitted by other routes have also been reported: aerosol infections among laboratory personnel [45], blood transfusions [46], and organ transplantation [47]. Transmission from an infected mother to her baby through breast milk is also suspected [5][48]. An example of a fatal TBEV infection following organ transplantation involved three patients who received organs from a single donor (two received a kidney and one received a liver). All the recipients developed encephalitis 17–49 days after the transplantation, which led to death. The presence of TBEV was confirmed using RT-PCR in the recipients and the donor, and sequencing confirmed the presence of the same virus strain. In this case, the course of the TBEV infection may have been complicated by pharmacological immunosuppression. It would be advisable for organ donors to be screened for TBEV, especially if they come from endemic regions [47].
5. Clinical Symptoms and Diagnosis
The incubation period of TBE ranges from 4 to 28 days [20]. The incubation after a foodborne infection is usually shorter, and up to 4 days [1]. Some studies have suggested a correlation between the length of the 3′ UTR of TBEV and the incubation period of the disease. In the case of viral strains with a 3′UTR sequence shorter than 200 nucleotides, the incubation period for suckling mice was longer than 5 days [13]. Other factors that may impact the incubation period are the viral load and subtype, the host’s innate and specific immunity, and flavivirus resistance gene structures [6]. The course of a TBEV infection varies and depends on the age and immune status of the infected person and the characteristics of the particular TBEV strain. Generally, an infection with TBEV can be symptomatic or asymptomatic. A symptomatic infection can be monophasic (with or without neurological symptoms) or biphasic, as it is in most patients. In the first stage of the biphasic course, nonspecific symptoms occur, such as a fever, headaches, and muscle pain lasting up to one week, and 70% of patients are diagnosed with leukopenia and thrombocytopenia. In the second phase, there are symptoms of encephalitis in the form of a persistent fever, headaches, insomnia, confusion, possible vomiting, a stiff neck, muscle pain, and paresis [1]. The second phase of the disease can also manifest as hemorrhagic syndrome [49]. In this phase of the disease, an increase in the white blood cell count, an elevated C-reactive protein (CRP) level, and a higher erythrocyte sedimentation rate (ESR) can be observed [50].
Tick-borne encephalitis is usually diagnosed clinically and serologically in the neurological phase of the disease [51][52]. Enzyme-linked immuno-sorbent assays are the current method of choice for the rapid detection of TBE-specific IgM and IgG antibodies in the sera of unvaccinated patients. However, IgM antibodies are not detected in serum or CSF in the early phase of the disease [53][54]. Specific IgM antibodies are usually detected in the serum when neurological symptoms occur, and the IgM response in CSF occurs later than it does in serum [55]. A study by Reusken et al. demonstrated the importance of IgM determination in serum and cerebrospinal fluid to diagnose a TBEV infection. An analysis of ELISA results showed a lack of IgG specificity. Additionally, the CSF/serum IgG antibody index can support a diagnosis in cases of chronic disease or when IgM has disappeared [56].
The use of molecular diagnostic methods, such as a TBE-specific PCR, allows for the identification of all TBEV subtypes in the early phase of the disease [57][58]. The molecular technique is of lesser importance in healthcare practice, since a diagnosis is usually required for patients with neurological symptoms of the disease. Viral RNA can be detected in blood or serum during the first phase of the infection, when the patients are asymptomatic or the symptoms are non-specific. After the onset of neurological symptoms, TBEV RNA is rarely detected in the blood or CSF [58], although persistent viremia has been reported in immunocompromised patients [59]. The duration of viremia is influenced by the environmental and body temperature. In large mammals, viremia is short-lived and only low virus titers are revealed. Birds also pass through a very short viremia stage [60].
This entry is adapted from the peer-reviewed paper 10.3390/jcm12206603
References
- Gritsun, T.S.; Lashkevich, V.A.; Gould, E.A. Tick-Borne Encephalitis. Antivir. Res. 2003, 57, 129–146.
- Chiffi, G.; Grandgirard, D.; Leib, S.L.; Chrdle, A.; Růžek, D. Tick-borne Encephalitis: A Comprehensive Review of the Epidemiology, Virology, and Clinical Picture. Rev. Med. Virol. 2023, 33, e2470.
- European Centre for Disease Prevention and Control. Tick-Borne Encephalitis. In Annual Epidemiological Report for 2020; ECDC: Stockholm, Sweden, 2022.
- Socha, W.; Kwasnik, M.; Larska, M.; Rola, J.; Rozek, W. Vector-Borne Viral Diseases as a Current Threat for Human and Animal Health—One Health Perspective. J. Clin. Med. 2022, 11, 3026.
- Ličková, M.; Fumačová Havlíková, S.; Sláviková, M.; Klempa, B. Alimentary Infections by Tick-Borne Encephalitis Virus. Viruses 2021, 14, 56.
- Bakhvalova, V.N.; Panov, V.V.; Morozova, O.V. Tick-Borne Encephalitis Virus Quasispecies Rearrangements in Ticks and Mammals. In Flavivirus Encephalitis; Ruzek, D., Ed.; InTech: Houston, TX, USA, 2011; ISBN 978-953-307-669-0.
- Stadler, K.; Allison, S.L.; Schalich, J.; Heinz, F.X. Proteolytic Activation of Tick-Borne Encephalitis Virus by Furin. J. Virol. 1997, 71, 8475–8481.
- Pulkkinen, L.I.A.; Barrass, S.V.; Domanska, A.; Överby, A.K.; Anastasina, M.; Butcher, S.J. Molecular Organisation of Tick-Borne Encephalitis Virus. Viruses 2022, 14, 792.
- Füzik, T.; Formanová, P.; Růžek, D.; Yoshii, K.; Niedrig, M.; Plevka, P. Structure of Tick-Borne Encephalitis Virus and Its Neutralization by a Monoclonal Antibody. Nat. Commun. 2018, 9, 436.
- Deviatkin, A.A.; Karganova, G.G.; Vakulenko, Y.A.; Lukashev, A.N. TBEV Subtyping in Terms of Genetic Distance. Viruses 2020, 12, 1240.
- Upstone, L.; Colley, R.; Harris, M.; Goonawardane, N. Functional Characterization of 5′ Untranslated Region (UTR) Secondary RNA Structures in the Replication of Tick-Borne Encephalitis Virus in Mammalian Cells. PLoS Negl. Trop. Dis. 2023, 17, e0011098.
- Ng, W.; Soto-Acosta, R.; Bradrick, S.; Garcia-Blanco, M.; Ooi, E. The 5′ and 3′ Untranslated Regions of the Flaviviral Genome. Viruses 2017, 9, 137.
- Morozova, O.V.; Bakhvalova, V.N.; Morozov, I.V. Heterogeneity of 3′-Untraslated Region of Genome RNA of the Tick-Borne Encephalitis Virus (TBEV) Strains Isolated from Ticks in the Western Siberia, Russia. Int. J. Biomed. Sci. 2007, 3, 206–210.
- Hirano, M.; Muto, M.; Sakai, M.; Kondo, H.; Kobayashi, S.; Kariwa, H.; Yoshii, K. Dendritic Transport of Tick-Borne Flavivirus RNA by Neuronal Granules Affects Development of Neurological Disease. Proc. Natl. Acad. Sci. USA 2017, 114, 9960–9965.
- Grard, G.; Moureau, G.; Charrel, R.N.; Lemasson, J.-J.; Gonzalez, J.-P.; Gallian, P.; Gritsun, T.S.; Holmes, E.C.; Gould, E.A.; de Lamballerie, X. Genetic Characterization of Tick-Borne Flaviviruses: New Insights into Evolution, Pathogenetic Determinants and Taxonomy. Virology 2007, 361, 80–92.
- Dai, X.; Shang, G.; Lu, S.; Yang, J.; Xu, J. A New Subtype of Eastern Tick-Borne Encephalitis Virus Discovered in Qinghai-Tibet Plateau, China. Emerg. Microbes Infect. 2018, 7, 1–9.
- Kovalev, S.Y.; Mukhacheva, T.A. Reconsidering the Classification of Tick-Borne Encephalitis Virus within the Siberian Subtype Gives New Insights into Its Evolutionary History. Infect. Genet. Evol. 2017, 55, 159–165.
- Kutschera, L.S.; Wolfinger, M.T. Evolutionary Traits of Tick-Borne Encephalitis Virus: Pervasive Non-Coding RNA Structure Conservation and Molecular Epidemiology. Virus Evol. 2022, 8, veac051.
- Sukhorukov, G.A.; Paramonov, A.I.; Lisak, O.V.; Kozlova, I.V.; Bazykin, G.A.; Neverov, A.D.; Karan, L.S. The Baikal Subtype of Tick-Borne Encephalitis Virus Is Evident of Recombination between Siberian and Far-Eastern Subtypes. PLoS Negl. Trop. Dis. 2023, 17, e0011141.
- Kaiser, R. The Clinical and Epidemiological Profile of Tick-Borne Encephalitis in Southern Germany 1994–98. Brain 1999, 122, 2067–2078.
- Mandl, C.W. Steps of the Tick-Borne Encephalitis Virus Replication Cycle That Affect Neuropathogenesis. Virus Res. 2005, 111, 161–174.
- Mansfield, K.L.; Johnson, N.; Phipps, L.P.; Stephenson, J.R.; Fooks, A.R.; Solomon, T. Tick-Borne Encephalitis Virus—A Review of an Emerging Zoonosis. J. Gen. Virol. 2009, 90, 1781–1794.
- Charrel, R.N.; Attoui, H.; Butenko, A.M.; Clegg, J.C.; Deubel, V.; Frolova, T.V.; Gould, E.A.; Gritsun, T.S.; Heinz, F.X.; Labuda, M.; et al. Tick-Borne Virus Diseases of Human Interest in Europe. Clin. Microbiol. Infect. 2004, 10, 1040–1055.
- Tkachev, S.E.; Chicherina, G.S.; Golovljova, I.; Belokopytova, P.S.; Tikunov, A.Y.; Zadora, O.V.; Glupov, V.V.; Tikunova, N.V. New Genetic Lineage within the Siberian Subtype of Tick-Borne Encephalitis Virus Found in Western Siberia, Russia. Infect. Genet. Evol. 2017, 56, 36–43.
- Kozlova, I.V.; Verkhozina, M.M.; Demina, T.V.; Dzhioev, Y.P.; Tkachev, S.E.; Karan, L.S.; Doroshchenko, E.K.; Lisak, O.V.; Suntsova, O.V.; Paramonov, A.I.; et al. Genetic and Biological Properties of Original TBEV Strains Group Circulating in Eastern Siberia. In Encephalitis; Tkachev, S., Ed.; InTech: Houston, TX, USA, 2013; ISBN 978-953-51-0925-9.
- Tkachev, S.E.; Babkin, I.V.; Chicherina, G.S.; Kozlova, I.V.; Verkhozina, M.M.; Demina, T.V.; Lisak, O.V.; Doroshchenko, E.K.; Dzhioev, Y.P.; Suntsova, O.V.; et al. Genetic Diversity and Geographical Distribution of the Siberian Subtype of the Tick-Borne Encephalitis Virus. Ticks Tick-Borne Dis. 2020, 11, 101327.
- Mandl, C.W.; Heinz, F.X.; Holzmann, H.; Kunz, C.; Ecker, M. Infectious CDNA Clones of Tick-Borne Encephalitis Virus European Subtype Prototypic Strain Neudoerfl and High Virulence Strain Hypr. J. Gen. Virol. 1997, 78, 1049–1057.
- Dumpis, U.; Crook, D.; Oksi, J. Tick-Borne Encephalitis. Clin. Infect. Dis. 1999, 28, 882–890.
- Chitimia-Dobler, L.; Lemhöfer, G.; Król, N.; Bestehorn, M.; Dobler, G.; Pfeffer, M. Repeated Isolation of Tick-Borne Encephalitis Virus from Adult Dermacentor Reticulatus Ticks in an Endemic Area in Germany. Parasites Vectors 2019, 12, 90.
- Krivanec, K.; Kopecký, J.; Tomková, E.; Grubhoffer, L. Isolation of TBE Virus from the Tick Ixodes Hexagonus. Folia Parasitol. 1988, 35, 273–276.
- Lichard, M.; Kozuch, O. Persistence of Tick-Borne Encephalitis Virus in Nymphs and Adults of Ixodes Arboricola and Its Transmission to White Mice. Acta Virol. 1967, 11, 480.
- Hubálek, Z.; Rudolf, I. Tick-Borne Viruses in Europe. Parasitol. Res. 2012, 111, 9–36.
- Riedl, H.; Kozuch, O.; Sixl, W.; Schmeller, E.; Nosek, J. Isolation of the tick-borne encephalitis virus (TBE-virus) from the tick Haemaphysalis concinna Koch. Arch. Hyg. Bakteriol. 1971, 154, 610–611.
- Kozuch, O.; Nosek, J. Transmission of Tick-Borne Encephalitis (TBE) Virus by Dermacentor Marginatus and D. Reticulatus Ticks. Acta Virol. 1971, 15, 334.
- Nosek, J.; Ciampor, F.; Kozuch, O.; Rajcáni, J. Localization of Tick-Borne Encephalitis Virus in Alveolar Cells of Salivary Glands of Dermacentor Marginatus and Haemaphysalis Inermis Ticks. Acta Virol. 1972, 16, 493–497.
- Bakhvalova, V.N.; Dobrotvorsky, A.K.; Panov, V.V.; Matveeva, V.A.; Tkachev, S.E.; Morozova, O.V. Natural Tick-Borne Encephalitis Virus Infection among Wild Small Mammals in the Southeastern Part of Western Siberia, Russia. Vector Borne Zoonotic Dis. 2006, 6, 32–41.
- Bakhvalova, V.N.; Potapova, O.F.; Panov, V.V.; Morozova, O.V. Vertical Transmission of Tick-Borne Encephalitis Virus between Generations of Adapted Reservoir Small Rodents. Virus Res. 2009, 140, 172–178.
- Wilhelmsson, P.; Jaenson, T.G.T.; Olsen, B.; Waldenström, J.; Lindgren, P.-E. Migratory Birds as Disseminators of Ticks and the Tick-Borne Pathogens Borrelia Bacteria and Tick-Borne Encephalitis (TBE) Virus: A Seasonal Study at Ottenby Bird Observatory in South-Eastern Sweden. Parasites Vectors 2020, 13, 607.
- Michelitsch, A.; Wernike, K.; Klaus, C.; Dobler, G.; Beer, M. Exploring the Reservoir Hosts of Tick-Borne Encephalitis Virus. Viruses 2019, 11, 669.
- Holding, M.; Dowall, S.D.; Medlock, J.M.; Carter, D.P.; McGinley, L.; Curran-French, M.; Pullan, S.T.; Chamberlain, J.; Hansford, K.M.; Baylis, M.; et al. Detection of New Endemic Focus of Tick-Borne Encephalitis Virus (TBEV), Hampshire/Dorset Border, England, September 2019. Euro Surveill. 2019, 24, 1900658.
- Jääskeläinen, A.; Tonteri, E.; Pieninkeroinen, I.; Sironen, T.; Voutilainen, L.; Kuusi, M.; Vaheri, A.; Vapalahti, O. Siberian Subtype Tick-Borne Encephalitis Virus in Ixodes Ricinus in a Newly Emerged Focus, Finland. Ticks Tick Borne Dis. 2016, 7, 216–223.
- Suzuki, Y. Multiple Transmissions of Tick-Borne Encephalitis Virus between Japan and Russia. Genes. Genet. Syst. 2007, 82, 187–195.
- Cerný, V. The Role of Mammals in Natural Foci of Tick-Borne Encephalitis in Central Europe. Folia Parasitol. 1975, 22, 271–273.
- Klaus, C.; Gethmann, J.; Hoffmann, B.; Ziegler, U.; Heller, M.; Beer, M. Tick Infestation in Birds and Prevalence of Pathogens in Ticks Collected from Different Places in Germany. Parasitol. Res. 2016, 115, 2729–2740.
- Avšič-Županc, T.; Poljak, M.; Matičič, M.; Radšel-Medvešček, A.; LeDuc, J.W.; Stiasny, K.; Kunz, C.; Heinz, F.X. Laboratory Acquired Tick-Borne Meningoencephalitis: Characterisation of Virus Strains. Clin. Diagn. Virol. 1995, 4, 51–59.
- Wahlberg, P.; Saikku, P.; Brummer-Korvenkontio, M. Tick-Borne Viral Encephalitis in Finland. The Clinical Features of Kumlinge Disease during 1959-1987. J. Intern. Med. 1989, 225, 173–177.
- Lipowski, D.; Popiel, M.; Perlejewski, K.; Nakamura, S.; Bukowska-Ośko, I.; Rzadkiewicz, E.; Dzieciątkowski, T.; Milecka, A.; Wenski, W.; Ciszek, M.; et al. A Cluster of Fatal Tick-Borne Encephalitis Virus Infection in Organ Transplant Setting. J. Infect. Dis. 2017, 215, 896–901.
- Kerlik, J.; Avdičová, M.; Musilová, M.; Bérešová, J.; Mezencev, R. Breast Milk as Route of Tick-Borne Encephalitis Virus Transmission from Mother to Infant. Emerg. Infect. Dis. 2022, 28, 1060–1061.
- Ternovoi, V.A.; Kurzhukov, G.P.; Sokolov, Y.V.; Ivanov, G.Y.; Ivanisenko, V.A.; Loktev, A.V.; Ryder, R.W.; Netesov, S.V.; Loktev, V.B. Tick-Borne Encephalitis with Hemorrhagic Syndrome, Novosibirsk Region, Russia, 1999. Emerg. Infect. Dis. 2003, 9, 743–746.
- Bogovic, P.; Lotric-Furlan, S.; Strle, F. What Tick-Borne Encephalitis May Look like: Clinical Signs and Symptoms. Travel Med. Infect. Dis. 2010, 8, 246–250.
- Ergunay, K.; Tkachev, S.; Kozlova, I.; Růžek, D. A Review of Methods for Detecting Tick-Borne Encephalitis Virus Infection in Tick, Animal, and Human Specimens. Vector Borne Zoonotic Dis. 2016, 16, 4–12.
- Taba, P.; Schmutzhard, E.; Forsberg, P.; Lutsar, I.; Ljøstad, U.; Mygland, Å.; Levchenko, I.; Strle, F.; Steiner, I. EAN Consensus Review on Prevention, Diagnosis and Management of Tick-Borne Encephalitis. Eur. J. Neurol. 2017, 24, 1214-e61.
- Holzmann, H. Diagnosis of Tick-Borne Encephalitis. Vaccine 2003, 21, S36–S40.
- Niedrig, M.; Vaisviliene, D.; Teichmann, A.; Klockmann, U.; Biel, S.S. Comparison of Six Different Commercial IgG-ELISA Kits for the Detection of TBEV-Antibodies. J. Clin. Virol. 2001, 20, 179–182.
- Günther, G.; Haglund, M.; Lindquist, L.; Sköldenberg, B.; Forsgren, M. Intrathecal IgM, IgA and IgG Antibody Response in Tick-Borne Encephalitis. Long-Term Follow-up Related to Clinical Course and Outcome. Clin. Diagn. Virol. 1997, 8, 17–29.
- Reusken, C.; Boonstra, M.; Rugebregt, S.; Scherbeijn, S.; Chandler, F.; Avšič-Županc, T.; Vapalahti, O.; Koopmans, M.; GeurtsvanKessel, C.H. An Evaluation of Serological Methods to Diagnose Tick-Borne Encephalitis from Serum and Cerebrospinal Fluid. J. Clin. Virol. 2019, 120, 78–83.
- Donoso Mantke, O.; Aberle, S.W.; Avšič-Županc, T.; Labuda, M.; Niedrig, M. Quality Control Assessment for the PCR Diagnosis of Tick-Borne Encephalitis Virus Infections. J. Clin. Virol. 2007, 38, 73–77.
- Saksida, A.; Duh, D.; Lotric-Furlan, S.; Strle, F.; Petrovec, M.; Avsic-Zupanc, T. The Importance of Tick-Borne Encephalitis Virus RNA Detection for Early Differential Diagnosis of Tick-Borne Encephalitis. J. Clin. Virol. 2005, 33, 331–335.
- Caracciolo, I.; Bassetti, M.; Paladini, G.; Luzzati, R.; Santon, D.; Merelli, M.; Sabbata, G.D.; Carletti, T.; Marcello, A.; D’Agaro, P. Persistent Viremia and Urine Shedding of Tick-Borne Encephalitis Virus in an Infected Immunosuppressed Patient from a New Epidemic Cluster in North-Eastern Italy. J. Clin. Virol. 2015, 69, 48–51.
- Pustijanac, E.; Buršić, M.; Talapko, J.; Škrlec, I.; Meštrović, T.; Lišnjić, D. Tick-Borne Encephalitis Virus: A Comprehensive Review of Transmission, Pathogenesis, Epidemiology, Clinical Manifestations, Diagnosis, and Prevention. Microorganisms 2023, 11, 1634.
This entry is offline, you can click here to edit this entry!







