Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Water Resources
膜分离技术已成为废水处理和海水淡化过程中生产清洁水的首选方法。这种偏好归因于该技术的高分离精度、能源效率、无二次污染和易于操作。膜污染是膜应用中的主要障碍,包括超滤 (UF)、微滤 (MF)、纳滤 (NF) 和反渗透 (RO)。膜结垢是工业废水预处理过程中一个特别严重的问题,导致水质差,运行成本增加。在使用膜的废水处理中,需要对结垢的形成和特性有透彻的了解,这有助于减缓膜结垢并实施适当的控制措施。作为回应,研究人员对膜污染进行了广泛的基础研究,试图阐明主要污垢、膜-污垢相互作用和潜在的污垢缓解技术。
- membrane fouling
- morphology
- roughness
- interactions
1. 膜的表征
膜和污垢之间的相互作用与膜表面特性有关,例如亲水性、粗糙度和电荷。开发高质量的防污膜可以减少污垢与膜表面之间的相互作用力,减缓膜污垢的发生。该技术有助于以高分辨率显示膜表面,表征膜表面信息的三维呈现,从而可以详尽地详细表达膜的表面特征。
1.1. 膜形态的表征
膜表面形态的可视化有助于理解膜的相关特性。AFM擅长可视化形态特征,允许在聚合过程中对膜表面功能层界面处发生的形态变化进行原位表征。在相反转过程中,利用AFM扫描液体环境中各种参数制备的纳滤膜或反渗透膜,可以原位观察表面功能层的更详细和系统的变化[16]。此外,AFM还可用于通过观察聚乙烯膜微观结构的变化来研究土壤微生物群落对聚乙烯膜的降解作用[19]。使用AFM,还可以观察膜的离子传输通道。通过原子力显微镜检查具有密集接枝离子簇的改性阴离子交换膜(AEM)材料可以揭示不同的离子传导途径,并证明修饰的AEM表现出出色的纳米相分离[20]。通过研究不同成像模式下使用AFM的纳滤膜表面,可以获得各种AFM成像模式特征[14]。AFM 中的攻丝模式允许在不破坏其形态的情况下精确测量柔软和精致表面的 3D 结构。该技术对于研究纳滤膜表面活性官能团层的界面聚合过程特别有用[6]。
显然,AFM可用于了解膜层表面的光滑度和均匀性;捕获膜表面的微观形态,包括表面缺陷、纳米级突起或凹陷;并提供膜表面形态的直观图像。因此,AFM不仅能够原位测量由水化学条件引起的表面形貌变化,而且还能够通过3D可视化图像理解和发现离子传输通道和纳米级形态。特别是,攻丝模式对薄而软的膜表面几乎是无损的。此外,AFM还可用于研究膜材料的表面电位信号,并将其与特定区域的物理化学性质叠加在一起。这些方面对于表征膜性能和理解膜制造至关重要。
1.2. 粗糙度的表征
膜的表面粗糙度是影响界面性能和结垢过程的关键因素。使用AFM不仅可以观察膜的表面形态,而且由于其沿x、y和z轴的三维测量能力,AFM还可以精确地表征膜表面/功能层的粗糙度,并提供详细的3D表面形貌图。AFM可用于了解各种类型膜的粗糙度,例如阳离子交换膜。在长期运行过程中,AFM可以精确测量粗糙度的变化,从而建立粗糙度与离子交换性能之间的关系。这增强了对阳离子交换膜的离子交换有效性和污染水平的监测[21]。
在膜改性过程中,在吸附交联过程中掺入特定的活性成分,如表面活性剂或高分子单体,可以增加表面粗糙度,改变膜功能层的结构。同样,用碳纳米管、金属氧化物和其他物质进行修饰可以改变膜的形态,增加粗糙度,扩大膜通道,从而改变膜的性能。使用AFM,可以观察到膜表面和通道的变化,并可以准确测量粗糙度。粗糙度是膜制造中的一个重要参数。结果一般表明,原膜表面粗糙度低、均匀、光滑。当添加改性剂或碳纳米管时,会形成纳米级改性结构,从而增加粗糙度。这种粗糙度的提高可以提高膜的防污性能、渗透蒸发性能、离子选择性,并调节膜的亲水性或疏水性。采用湿相转化法并添加表面活性剂(Pluronic F127)制造的不对称聚苯乙烯膜增加了膜的表面粗糙度并加强了膜通道,从而显着增强了膜的渗透蒸发性能[22]。同样,使用AFM对多壁碳纳米管(MWCNT)分散PS纳滤膜[17]的分析表明,原始PS膜的表面光滑均匀,而MWCNTs的加入增加了表面粗糙度,使结构更加明显,从而提高了PS膜的透氢性。使用AFM进行的粗糙度测量表明,等离子体处理和表面酸化也增加了离子交换膜的粗糙度,从而实现了更多的离子交换,并促进了具有优异性能的离子交换膜的制备[23]。
然而,研究表明,对于粗糙的表面,纳米级改性结构具有更好的防止膜污染的倾向,但当修饰结构过大时,它们会加剧膜污染。这些相关的见解可以通过使用AFM精确测量粗糙度来获得。此外,通过共聚和接枝方法对膜表面进行改性可以增加膜的粗糙度,AFM可以原位测量膜改性过程中的粗糙度变化。当使用AFM表征亲水性聚合物官能化聚砜(PSF)共混膜时[24],研究人员发现,添加4VP侧链增强了改性膜的表面粗糙度。在另一项研究中,用氧化钛化合物改性的PSF膜[25]具有更高的表面粗糙度,并且这些改性膜表现出优异的亲水性和防污性能。表面活性剂聚多巴胺和3-(N,N-二甲基肉豆蔻酰基)丙烷磺酸酯[26]的简单编码可以实现聚醚砜(PES)超滤膜的防污性能。用AFM扫描这些改性膜可以得到粗糙度参数Rq和Ra,表明改性膜显著缓解了通量下降,增强了防污性能。
利用AFM精确测量膜表面粗糙度,可以探索改性膜表面哪种粗糙度更耐污染,从而实现膜性能调整。尽管许多研究表明,粗糙度的增加可能导致膜污染的倾向增加[21,24],但其他研究结果表明,在纳米级颗粒中添加微米级以增加表面粗糙度(类似于荷叶仿生结构)可以减少膜污染[17,23].这种差异主要是因为单个粗糙度参数不足以概括膜表面结垢的复杂性。建立用AFM测量的粗糙度R与粗糙度指数H之间的关系,可以更快、更准确地评估膜表面粗糙度[27]。此外,整体评估应将AFM与各种其他技术相结合,以仔细检查膜表面特性,例如表面电位,亲水性/疏水性,官能团和污垢特性。通过这种对表征结果的综合判断,可以彻底分析表面粗糙度与膜结垢之间的关系。建立AFM测得的膜表面粗糙度与膜表面电位、官能团等的关系,可以更好地帮助优化膜的亲水性/疏水性、渗透选择性、离子选择性和防污性能,为膜界面的设计和优化提供指导。
1.3. 膜通道的测量
膜通道对于膜的过滤性能至关重要,因为这些通道的大小和结构直接影响膜的选择性和渗透通量,这与膜的权衡效应有关。AFM已被用于检测改性膜的各种表面参数,包括膜通道的结构和孔径。这些研究人员[18,28,29,30,31,32]使用AFM表征了改性膜的表面,发现改性剂的沉积过程使膜表面更光滑,消除了小尺度的粗糙特征,减小了孔径,并降低了对污垢的敏感性。例如,Kim等[28]使用氧化石墨烯(GO)结合等离子体增强原子层沉积(ALD)技术实现了纳滤膜的原子级表面功能化。使用一种新的数据分析方法[18]将AFM与“孔隙重建技术”相结合,以评估膜通道结构,包括尺寸、形状和层间距离。获得的膜通道信息对于膜脱盐中的渗透选择过程至关重要,也可以作为评估膜污染倾向的关键因素。AFM可以精确测量通过沉积方法修饰的氧化锌涂层铝膜通道的层间距离[29],识别涂有氧化锌纳米结构(ZnO NW)的超亲水铜网膜的膜通道结构,用于油水分离[30],获取有关使用分子层沉积(MLD)技术创建的改性海水淡化纳滤膜中膜通道形状的信息[[31],并获得壳聚糖和聚苯乙烯磺酸盐改性聚酰胺微滤膜中膜通道的3D形状信息,采用逐层(LBL)沉积法制备[32]。
结合从AFM获得的表面和横截面图像,可以构建膜通道的三维图像,提供有关通道的大小、形状和排列的详细信息,并更准确地预测膜通道堵塞和膜污染的程度。研究表明,与分布不均匀相比,均匀分布的孔隙结构可能会降低结垢的风险。均匀膜中孔隙的系统分布可以增强对污垢的拦截能力[33]。值得注意的是,膜通道的几何形状极大地影响了膜的污染。通常,狭缝状孔隙引起的污垢强度低于圆形孔隙引起的污染强度[34]。此外,AFM可以准确地呈现真实液相环境中膜通道堵塞的程度和膜结垢的状态。相比之下,SEM需要在干燥和真空条件下进行测量,这可能会导致结果失真和孔隙信息不准确。因此,通过AFM获得的膜通道数据对于跟踪膜污染轨迹以及评估和改善膜性能至关重要。
如前所述,使用AFM表征不同的膜可以更全面地观察三维表面形态、膜粗糙度测量、膜通道评估,以及对膜和改性膜的材料特性和应用性能进行整体评估。如图 1 所示,本节对上述文献中引用的通过 AFM 进行改良膜表征的不同模式、特征和结果进行了分类和总结。AFM还广泛用于表征各种材料的改性膜,例如改性的Langmuir-Blodgett(LB)薄膜[35]、形状独特的改性嵌段共聚物微滤膜[36]、沸石填充聚醚砜膜[37]、改性的Carbosep M5陶瓷膜[38]、创新的带正电荷的纳滤膜[39]、用于油水分离的有机膜[40]和用于灰水处理的复合陶瓷微滤膜[41]。这种技术(原子力显微镜)已成为功能膜设计和制造的有力工具。
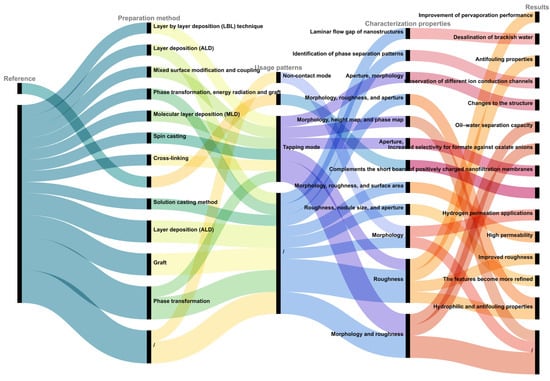
图 1.不同模式下改性膜的AFM表征的不同方面和结果。
2. 污染物的表征
在基于膜的技术和其他技术中会遇到不同类型的膜污垢;因此,使用AFM在微观水平上仔细检查污染物的特性至关重要。AFM的纳米级分辨率可以直接观察膜表面的污染物形态和结构。因此,AFM可用于实时监测污染物在各种环境条件下的吸附和粘附。活微生物在代谢过程中的形态变化可以通过敲击或非接触模式记录,这对其他技术来说是一个挑战。以这种方式利用AFM的功能可以增强对膜污染机制的理解。表征污染物有助于更好地理解膜污染原理,并为膜过滤系统的设计、操作和维护提供必要的指导。这是研究膜污染机制的重要因素。
2.1. Organic Contaminants
Natural organic matter (NOM) is the primary contaminant in wastewater. It is a complex heterogeneous system comprising diverse organic molecules [45], such as humic substances, polysaccharides, and proteins, which can all affect the membrane performance. Observations using AFM in aquatic environments have revealed that natural polysaccharide sodium alginate (SA) predominantly exists as single helical chains, with diameters of approximately 0.2–0.3 nm [42]. Scanning humic acid sodium (HA)-contaminated mica surfaces with AFM has uncovered spherical particles and aggregates, featuring colloidal diameters under 100 nm and heights from 0.5 to 7 nm [46]. In studies on protein membrane fouling, most protein molecules have been observed as monomers on mica surfaces [47]. Extracellular organic matter (EOM) can lead to severe ultrafiltration membrane fouling. AFM enables the observation of the aggregation and blockage behaviors of pollutants on the membrane surface [48], and evaluates the effects of cleaning/pre-treatment [49]. Utilizing AFM technology aids in further understanding the impact of natural organic matter (NOM) on membrane performance during water treatment processes, thereby laying the foundation for mitigating organic membrane fouling.
2.2. Biological Contaminants
In addition to typical organic contaminants, biological contaminants can impair membrane treatment efficiency in water treatment processes. Escherichia coli is a common pathogenic microorganism that compromises the safety of water resources and drinking water. Researchers have used AFM to investigate the morphological changes in E. coli on membrane surfaces under varying pH conditions [43] and correlate it with membrane filtration and cleaning [50]. In addition, AFM has been used to examine changes in the morphology of antibiotic-resistant E. coli on membrane surfaces during photocatalytic Fenton water treatment [51]. Recently, owing to the potential problem of microalgae in water treatment processes, particularly in membrane treatment, AFM has been applied extensively to study microalgal cell morphology and nanomechanical properties on membrane surfaces. High-speed atomic force microscopy (HS-AFM) has been employed to analyze Chlorella vulgaris treated with electrocoagulation flotation (ECF) [52]. Another study used AFM to determine the energy required to disrupt individual microalgae cells [53]. Guidance could be offered for alleviating biological fouling caused by microalgae. For the living microbial cells, AFM-based single-cell force spectroscopy (AFM-SCFS) has significant value for characterizing the structure, mechanical properties, and molecular activity of individual living microbial cells [54]. The technique can measure the mechanical properties of a single microorganism, quantify individual microorganism adhesion forces, and perform structural imaging of microbial behavior while simultaneously sensing microbial activity in real-time. Wang et al. [55] employed AFM to explore the dynamic effects of various environmental factors on microorganisms and membrane surface interactions at a molecular scale. This provides a research basis for the effective inhibition of biological foulants on membrane surfaces.
AFM-SFCS allows sensitive measurements of the mechanical properties of individual molecules. This allows researchers to gain insight into the mechanical properties of individual molecules such as stretching, deformation, and fracture, which is important for understanding the properties of biomolecules, polymers, and other materials. Nevertheless, AFM-SCFS has not reached maturity yet and still presents several technical challenges. Based on our group’s research on AFM-SFCS in the environmental field, we found that this technology faces the following problems in its application. First, the adhesion of live single cells to the probe tip is difficult and requires the selection of suitable adhesives for cell immobilization. Additionally, assessing the viability of single cells on the probe tip after attachment is challenging, prompting researchers to explore more advanced methods for examining post-adhesion cell viability. The morphology of single cells is not consistent; it encompasses rod or spherical shapes and other irregular shapes. During the adhesion process, it is crucial to consider different adhesion positions and variations in contact areas with the measurement surface to prevent inconsistencies in the recorded force magnitudes. Furthermore, even when live cells successfully adhere, it is difficult to maintain consistent single-cell activity at the probe tip (considering the different activity levels of young and aged cells at various stages). Ongoing investigation and refinement of AFM-SCFS techniques is anticipated to address these issues in the near future. Researchers could, therefore, gain better understanding of the characteristics of biological contaminants using AFM technology, further elucidate the membrane fouling process, guide biofouling removal, and offer theoretical support and practical guidance for the development of long-lasting antifouling membrane materials and superior biofouling control strategies.
2.3. Emerging Contaminants
Emerging contaminants in wastewater treatment processes, such as microplastics, antibiotics, and endocrine-disrupting compounds (EDCs) have been attracting increasing academic attention at national and international levels. As a high-resolution tool, AFM enables a more detailed examination of the physical properties of microplastics [56]. For instance, Melo-Agustín et al. [57] employed AFM for morphological analysis of microplastic surfaces, discovering that polyethylene (PE) microplastic surfaces exhibit higher levels of roughness than polypropylene (PP) microplastic surfaces. This observation suggests that PE is more susceptible to degradation than PP, potentially leading to greater contaminant adsorption. Chen et al. [58] introduced a method that combines AFM with infrared spectroscopy (AFM-IR) to characterize nanoplastics (NPs). This hybrid AFM technique can identify and image the chemical composition of nanoplastics at a high spatial resolution (20–100 nm), thereby offering a novel approach to NP characterization. However, the large specific surface area of microplastics often causes them to function as ‘carriers’ of other contaminants during water treatment processes, which exacerbates pollution. For instance, Zhang et al. [59] employed AFM to determine the interaction forces between NPs (hematite and corundum) and Escherichia coli cells, gaining further understanding of the membrane fouling mechanism of microplastics.
Additionally, antibiotics are frequently occurring emerging pollutants in aquatic environments, and even at trace concentrations, antibiotics in wastewater can adversely affect human health. AFM can effectively characterize the morphology and interaction forces of antibiotics on the membrane surface, thereby enhancing the efficiency of membranes in intercepting them. For instance, Liu et al. [60] used AFM to investigate the adsorption of EDCs on nanofiltration membrane surfaces, subsequently enhancing the EDC removal rate by preparing modified nanofiltration membranes. Wu et al. [61] attached sulfamethoxazole (SMX), a representative antibiotic, to an AFM tip to measure the SMX adhesion force distribution. Their study revealed the adhesion mechanism of SMX and, potentially, that of other sulfonamide antibiotics at a molecular level from both experimental and theoretical viewpoints. Researchers have also examined the impact of microplastics on antibiotic transport during sand filtration [44] by grafting ciprofloxacin (CIP) and sulfamethoxazole (SMX) onto AFM probes to determine the adhesion forces between representative microplastics (PS and PE) and quartz sand. Their study explored the mechanism of microplastics that enhances antibiotic transport in sand filtration systems from the perspective of molecular interactions.
3. Microscopic Identification of Membrane Fouling Processes under Changing Factors
The previous text, respectively, introduced the membrane and contaminants observed by AFM. However, membrane fouling is a complex fouling process influenced by multiple factors. Therefore, our research team conducted abundant research on the effects of changes in the ionic concentration, pH, and time on membrane fouling using AFM. By employing AFM, researchers can monitor the morphological changes in contaminants under various environmental conditions, facilitating real-time observation of the adsorption process on membrane surfaces. Once contaminants are adsorbed, alterations in environmental factors (ionic conditions, pH, membrane surface properties, and time) could cause varying fouling morphologies and characteristics compared with their initial states.
Ionic conditions: Our research team [62,63,64,65] used AFM to study the effects of different valence ions on membrane fouling of NOMs. Using AFM force measurements, morphology characterization, and other technical methods, the effect of monovalent ions such as Na and K on organic compounds was found to be based on their charge and structure. However, the effect of divalent ions such as Ca++2+ and Mg2+ on organic compounds also included complexation. Among them, it is closely related to the special functional groups, types, and structures of NOMs. Miao et al. [68,69] employed AFM to investigate the effects of Na, Mg+2+, and Ca2+ on HA fouling through HA membrane fouling experiments. These authors observed that membrane fouling intensified at lower Ca2+ or Mg2+ concentrations and significantly decreased at substantially higher Ca2+ or Mg2+ concentrations, albeit with the two ions having different mechanisms.
pH: We investigated changes in membrane fouling under different pH conditions using AFM [64]. The results showed that at a pH range of 4–6, the adherence of polysaccharide fouling, and its reversibility, depended on the functional groups. When the organics were rich in –COOH, an increase in pH reduced their deposition on the membrane surface and alleviated adsorptive fouling and irreversibility. For the –NH2 functional group, an increase in pH led to more severe polysaccharide fouling owing to a lower degree of protonation, and the resulting fouling was highly irreversible. Modification using GO alleviated the adsorptive fouling of these two polysaccharides on PVDF; however, the extent of alleviation depended on the abundance of functional groups on the polysaccharides.
Time: Interestingly, we found that time changes could affect membrane fouling [55]. We studied the pollution behavior of three selected model foulants at different adsorption times. For the SA-Ca2+ system, a longer adsorption time slightly increased the adsorption capacity of SA but significantly reduced its reversibility. With regards to BSA-Ca2+, the extended time did not change the amount of BSA deposited on the membrane surface but led to more residual BSA after cleaning. Similarly, in the HA-Ca2+ system, the adsorption time had almost no effect on the adsorption amount of HA but reduced its reversibility. Duration had a significant effect on the quantity and reversibility of membrane fouling, depending on the chemical properties of the membrane. Therefore, the AFM measurement results indicate that the longer the adsorption time, the denser the fouling layer and the stronger the interaction force between the fouling membranes.
Other factors: We also used AFM to study the effects of voltage on the fouling of a novel polypyrrole (PPy) and stainless steel mesh conductive composite membrane [67]. We found that the PPy ‘cauliflower’ structure expanded as the applied voltage increased (Figure 7), and the corresponding roughness of the feature area gradually decreased from 5.91 to 4.34 nm. This result could probably be ascribed to the delocalized conjugated electron carrier in the conducting polymer moving along the polymer chain under an external electric field, which changed the dipole moment of the PPy molecules. Such change caused changes in the conformation and intermolecular arrangement of the PPy molecules, resulting in the expansion of surface morphology and, thereby, decreasing the roughness.
In addition to the these membrane fouling investigations, Arkhangelsky et al. [70] employed AFM to investigate the membrane-cleaning process and examined the influence of different cleaning agents on membrane surfaces. Analysis employing AFM revealed that the sodium hypochlorite (NaOCl) cleaning agent affected the contaminants and the membrane, leading to partial organic matter destruction and a modified membrane surface. In contrast, sodium hydroxide (NaOH) treatment completely destroyed the proteins, yielding a smooth surface with minimal residual matter. Similarly, using AFM to examine the fouling behavior of BSA on the membrane, it was found that pre-chlorination significantly mitigated membrane fouling, whereas pre-ozonation oxidation exacerbated it [66]. These studies leveraged AFM technology to characterize the morphology of common contaminants on membrane surfaces and to elucidate the alterations and characteristics of the membrane fouling surface morphology under various conditions, such as time and pH. This information provides a theoretical basis for the mechanism of converting irreversible fouling into reversible fouling, and effectively informs membrane fouling control strategies.
4. Measurement of Interactions in Membrane Fouling
In the membrane treatment process, the micro-interaction between membranes and foulants significantly affects the formation of membrane fouling. The AFM technology offers valuable insights into the characteristics of foulants and membrane–foulant interactions, which could be leveraged to develop more effective strategies for preventing and controlling membrane fouling. Such strategies include optimizing membrane materials and surface modifications, enhancing pre-treatment processes, and creating innovative cleaning and regeneration technologies, which could reduce operational costs and prolong the lifespan of the membranes. The interaction force between foulants and the membrane is crucial for determining the efficiency of membrane fouling removal. Nanomechanical measurements using AFM and the quantification of interfacial interaction forces during membrane fouling provide essential information on the nanomechanical properties of foulants and membrane surfaces. Such information is critical for understanding membrane fouling.
Combining AFM with BSA-adsorbed SiO2 microsphere colloidal probes to investigate membrane surface fouling in the presence of BSA [71]. These authors observed that the adhesion force between PVDF-BSA were −1.5 nN, whereas the adhesion force between BSA-BSA were nearly zero, suggesting that BSA fouling behavior was predominantly influenced by the physicochemical interaction between the membrane polymer and BSA. Membrane-coated colloidal probes made of SiO2 microspheres coated with PP/PA are utilized in AFM to investigate the mechanism of membrane fouling caused by HA [72]. Force measurements showed that the interaction between the membrane and foulants was the primary factor contributing to the membrane fouling behavior. In a study of membrane fouling involving HA and SA [73], indention and retraction curves obtained from force spectroscopy measurements using an AFM probe modified with silicon nitride were used to characterize the surface stiffness and adhesive properties of fouled and clean membranes. These authors discovered that bacterial cells neither adhered to nor penetrated the organic fouling layer but, instead, traversed the thin foulant layer and directly adhered to the membrane surface.
To further understand and clarify the fouling behavior of HA and SA on membranes, Miao et al. [75] used AFM in conjunction with PVDF and foulant-coated probes to investigate the intermolecular forces between the membrane and contaminants (SA, HA, or HA/SA mixtures), as well as the forces between the contaminants themselves. Owing to the strong interaction between the hydroxyl groups in SA and PVDF, the adhesion force between PVDF and SA was more than double that of PVDF-HA. The formation of organic fouling on membranes can be studied by adsorbing the corresponding EfOM components onto the surface of PVDF microspheres sintered on cantilevers prepared to form EfOM-coated colloidal probes [76]. Using AFM, these authors demonstrated that the adhesion force between PVDF and different parts of the EfOM follow the order PVDF-TPI (affinitive) < PVDF-HPO (hydrophobic) < PVDF-HPI (hydrophilic). Several researchers have examined membrane fouling under the combined action of BSA and HA [74]. They created colloidal probes with BSA directly attached to the probe tip and employed AFM-based chemical force spectroscopy for adhesion force measurements. Furthermore, employing AFM to examine the interaction energy between polyvinyl chloride (PVC) membranes and three water contaminants, namely HA, BSA, and dextran (DEX) [77], helps in revealing the complex mechanisms of related membrane fouling.
Analyzing the AFM results for the interaction forces between individual and multiple organic contaminants with membranes has led to the following conclusions. Generally, the interaction between membranes and foulants is stronger than the interaction forces among the foulants themselves. HA adsorption significantly decreases the BSA adhesion force on hydrophobic surfaces. The fouling rate of PVC membranes follows the order of DEX > BSA > HA, demonstrating that selecting suitable pretreatment processes to remove specific foulants can effectively control polyvinyl chloride membrane fouling. Owing to the strong interaction between the hydroxyl groups in SA and PVDF, SA, rather than HA, has been identified as the primary cause of PVDF membrane fouling. This implies that the pretreatment process for removing SA is crucial in controlling PVDF membrane fouling. It suggests that employing appropriate methods, such as pretreatment, membrane modification, or cleaning, to reduce the hydrogen bonding interactions between PVDF and foulants is an effective strategy for reducing adhesion forces. Choosing pretreatments that convert HPI and HPO fractions into TPI fractions is essential for controlling PVDF membrane fouling during secondary effluent filtration. Therefore, AFM force measurements provide valuable information for selecting membrane modifications, feedwater pretreatment, and cleaning technologies in wastewater treatment and desalination.
最近,AFM越来越多地用于研究各种污垢和膜之间的相互作用力。AFM的单力光谱曲线用于评估膜和污垢之间的相互作用,是调整改性膜性能的关键参数。该技术不仅可以测量硬物之间的相互作用力,还可以测量涉及较软实体的相互作用力,例如在预处理的改性膜和相关膜污垢之间的相互作用力测量中[78]。学者们利用AFM的单力谱曲线阐明了阴离子交换膜(AEM)中阴离子聚丙烯酰胺(APAM)诱导的电渗析结垢机制[79]。除了膜之外,AFM还可以表征各种涂层与其他物质之间的相互作用力,例如不同溶液中的气泡[80]、溶解有机物[81]和沥青涂层的球形颗粒之间的相互作用。它还可以测量活微生物与膜之间的相互作用,这是至关重要的,因为活细胞在外力作用下分泌外泌体,并且它们与膜的相互作用力在应激条件下会发生变化。使用AFM测量这些力可以更准确地反映膜表面的生物污染。Yumiyama等[82]直接测量了单个酵母细胞之间的相互作用力。学者们还研究了酵母细胞和微泡(MB)之间的粘附力[83]。这些研究证明了AFM的单力谱曲线在测量污垢和膜之间的相互作用力方面的效用,可用于评估膜-污垢界面的特征和相互作用。这有助于开发高性能改性膜和更有效的膜清洁方法。
5. 膜污染相互作用的建模或分析
在了解膜污染过程时,杂质的特性(如尺寸、形状、电荷特性和化学稳定性)和膜材料的属性(如孔径、表面粗糙度、化学稳定性和电荷特性)显着影响杂质与膜之间的相互作用模式。某些杂质可能与特定的膜材料发生更强烈的相互作用,可能导致严重的膜污染。例如,带正电荷的杂质可以强烈吸附在带负电荷的膜材料上,形成污垢层。相反,如果膜材料和杂质之间由于带电荷而产生排斥力,则结垢程度可能会降低。离子组成也会对污垢与污垢的相互作用产生重大影响[84]。因此,对这种相互作用的建模和分析可以为预测和优化膜过程的性能提供关键的见解。
通过将AFM力测量的结果与某些现有的理论或模型相结合,例如扩展的Derjaguin-Landau-Verwey-Overbeek(XDLVO)理论[85,86]和Hermia模型[87,88],可以预测粒子接近膜表面时力的作用方式,以及它们对粒子吸附行为的影响。这允许根据分子特征预测膜污染。Wang等[62]采用XDLVO模型计算了不同离子强度下PVDF膜与有机物的相互作用能,发现随着Na浓度的增加,路易斯酸碱(AB)力值逐渐减小。AB力与颗粒和膜的化学官能团有关[89]。结果表明,离子强度的增加增强了膜与有机物之间的AB相互作用,这与离子强度的增加,膜表面吸附的有机物总量是一致的。不仅如此,XDLVO相互作用和表面粗糙度可能共同影响新兴的多功能纳米杂化物在环境中的运输和命运[90]。此外,这些作者[91,92]发现,XDLVO理论计算的结果与AFM分析结果一致,表明AFM力-距离曲线可以有效地验证计算结果,并且AFM在测量膜和污垢之间的相互作用方面具有高度的可靠性。整合AFM力测量技术来分析膜过滤过程中的阻塞机制[87]有助于更好地了解膜污染现象并制定有效的污染预防策略。+
Hermia模型[88]通过拟合表观抗污性与膜过滤时间之间的关系,确定了由不同阻塞机制引起的污垢类型[93]。Huang等[94]基于Hermia模型开发了统一膜污染指数(UMFI)。通过直接测试商用膜,UMFI可以量化膜污染的可能性,这对于评估在不同水处理规模的低压膜(LPM)中观察到的污染非常有用。AFM力测量技术可用于验证这些已建立的模型,有助于加深对膜封闭机制的理解。
应该注意的是,尽管 XDLVO 理论和 Hermia 模型提供了有用的见解,但它们并不涵盖所有类型的污垢行为。这些理论和模型更适合在稳定条件下进行预测,而实际的水处理过程通常面临着更复杂、动态和不断变化的条件。新的研究旨在整合实验和理论方法,以更全面地理解和预测杂质与膜材料之间的相互作用。例如,AFM和其他纳米级表征技术用于直接观察和测量杂质和膜之间的相互作用,而分子动力学模拟和量子化学计算则用于在原子尺度上理解这些过程。分析和模拟不同杂质和膜材料之间的潜在相互作用是膜科学和工程的关键因素。需要结合各种实验和理论方法,才能获得全面而深入的理解。在此过程中,AFM可以通过测量污染物与膜表面之间的相互作用力来预测污染物的吸附趋势。此外,AFM还可以实时监测污染过程,例如污染物在膜表面的吸附、扩散和聚集。将AFM与相关理论和模型相结合,有助于进一步探索膜污染过程和预测膜污染趋势。
This entry is adapted from the peer-reviewed paper 10.3390/membranes14020035
This entry is offline, you can click here to edit this entry!
