Wetting the surface area of an electrode material as completely as possible is desirable to achieve optimum specific capacity of an electrode material. Keeping this surface area utilized even at high current densities and even when inside small pores is required for high capacitance retention. The addition of surfactants at small concentrations to aqueous supercapacitor electrolyte solutions has been suggested as a way to improve performance in terms of capacitance, capacitance retention at increased current density and stability. Effects are pronounced with carbon materials used in electrochemical double-layer capacitors; they are also observed with redox materials.
- supercapacitor
- ultracapacitor
- electrochemical capacitor
- surfactant
1. Introduction
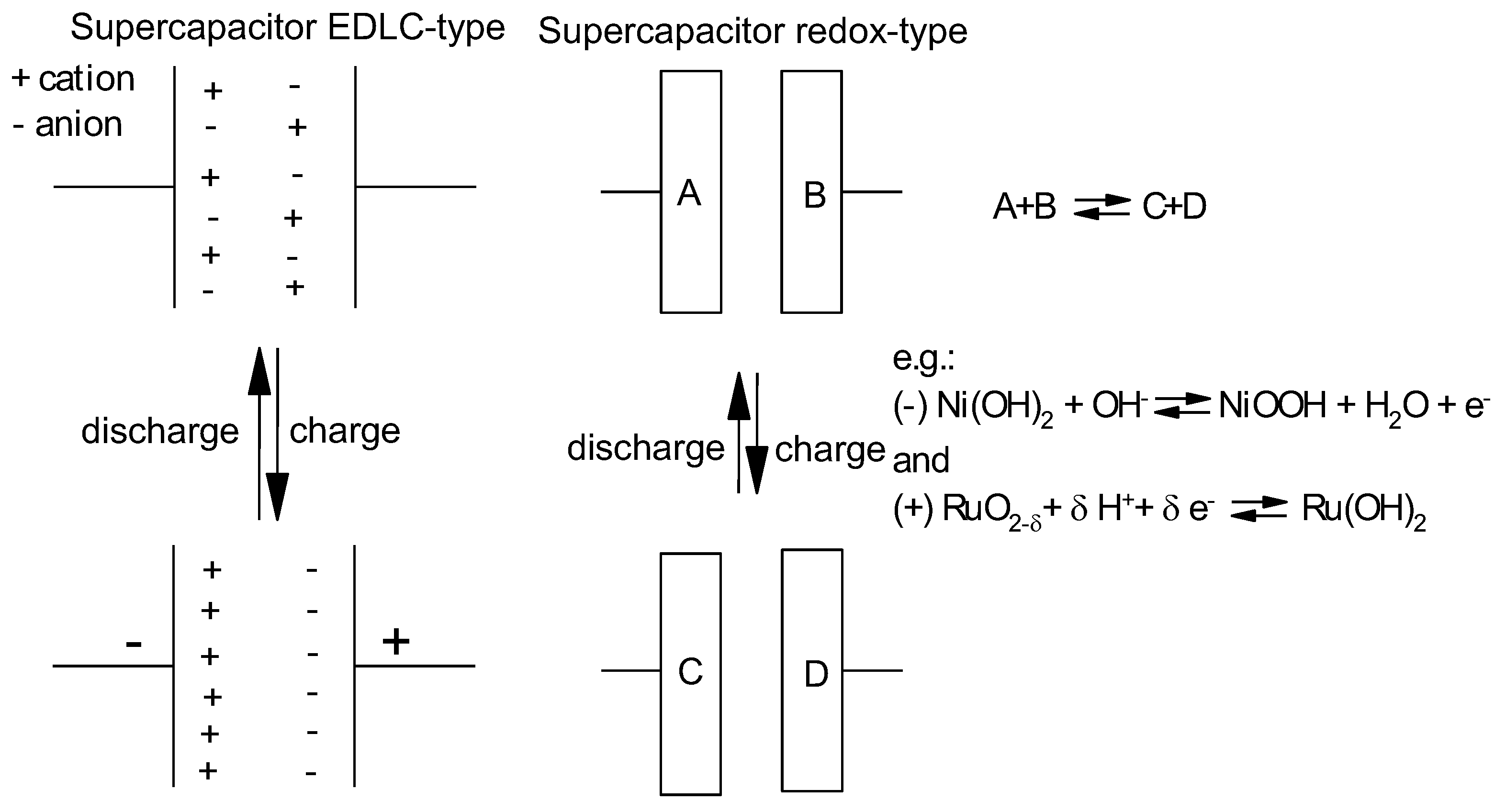
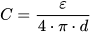
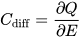
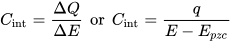
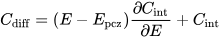
2. Approaches to Increased Material Utilization
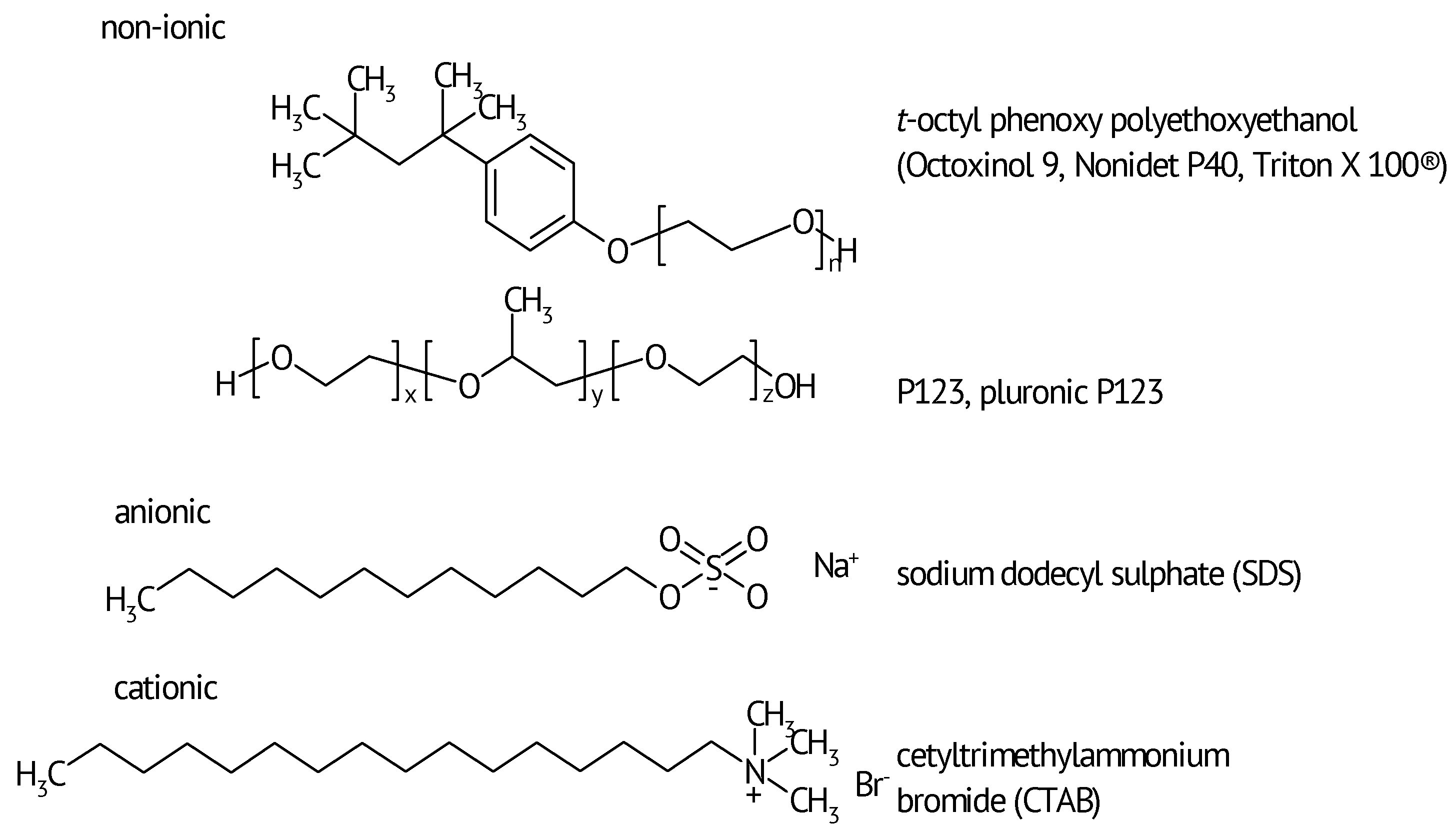
3. Results and their current understanding
Fic et al. [58][59][60] reported on the effect of various surfactants added at 5 mM concentration into the 6 M KOH electrolyte solution of a symmetric supercapacitor of the EDLC-type with activated carbon electrodes. Higher surfactant concentrations were inefficient because of micelle formation. Best improvements including enhanced capacitance at higher current density, i.e., major improvement of capacitance retention with growing current density, slower self-discharge (of a complete device in terms of e.g. cell voltage or a single electrode in terms of electrode potential) and increased stability were found with non-ionic Triton X-100. The claimed main effect was lower surfface tension of the aqueous phase. Unfortunately, these statements appear to be slightly inconclusive. Upon closer inspection at lowest current density, addition of any surfactant did not show an increase of specific capacitance. Accordingly a simple wetting enhancement is not the full explanation – if any explanation at all because it should have resulted in higher values of specific capacitance even at lowest scan rates. The significantly improved capacitance retention with the studied AC electrode material suggests instead better utilization of inner pore surface area than without added surfactant. How this can be cause by addition of a surfactant remains to be studied. This conclusion also applies to the noted improved stability.
With redox-active electrode materials, e.g. metal chalcogenides, reported effects are so far small, observations are rare.
This entry is adapted from the peer-reviewed paper 10.3390/batteries10010004
References
- Dubal, D.P.; Wu, Y.P.; Holze, R. Supercapacitors: From the Leyden jar to electric busses. Chemtexts 2016, 2, 13.
- Khorate, A.; Kadam, A.V. An overview of patents and recent development in flexible supercapacitors. J. Energy Storage 2022, 52, 104887.
- Peljo, P.; Girault, H.H. Electrochemical potential window of battery electrolytes: The HOMO-LUMO misconception. Energy Environ. Sci. 2018, 11, 2306.
- Holze, R. Overoxidation of Intrinsically Conducting Polymers. Polymers 2022, 14, 1584.
- Xie, X.; Holze, R. Meaning and Determination of Electrode Surface Area. Available online: https://encyclopedia.pub/entry/41569 (accessed on 2 May 2023).
- Vielstich, W.; Schmickler, W. Elektrochemie II: Kinetik Elektrochemischer Systeme; Haase, R., Ed.; Steinkopff: Darmstadt, Germany, 1976.
- Gileadi, E.; Kirowa-Eisner, E.; Penciner, J. Interfacial Electrochemistry; Addison Wesley: London, UK, 1975.
- Holze, R.; Schneider, J.; Hamann, C.H. Eine neue Methode zur Untersuchung der Elektrosorption reaktiver Verbindungen. Ber. Bunsenges. Phys. Chem. 1988, 92, 1319–1325.
- Doss, K.S.G.; Kalyanasundaram, A. Effect of surface active substances on the capacity of the electric double layer. Proc. Indian Acad. Sci. 1952, 35A, 27–33.
- Breyer, B.; Hacobian, S. Tensammetry: A Method of Investigating Surface Phenomena by AC Current Measurements. Aust. J. Sci. Res. Ser. A 1952, 5, 500–520.
- Plambeck, J.A. Electroanalytical Chemistry; Wiley: New York, NY, USA, 1982.
- Holze, R. Landolt-Börnstein: Numerical Data and Functional Relationships in Science and Technology, New Series, Group IV: Physical Chemistry, Volume 9: Electrochemistry, Subvolume A: Electrochemical Thermodynamics and Kinetics; Martienssen, W., Lechner, M.D., Eds.; Springer: Berlin, Germany, 2007.
- Jehring, H. Elektrosorptionsanalyse Mit der Wechselstrompolarographie; Akademie-Verlag: Berlin, Germany, 1975.
- Randin, J.P.; Yeager, E. Differential Capacitance Study of Stress-Annealed Pyrolytic Graphite Electrodes. J. Electrochem. Soc. 1971, 118, 711–714.
- Randin, J.P.; Yeager, E. Differential capacitance study on the basal plane of stress-annealed pyrolytic graphite. J. Electroanal. Chem. 1972, 36, 257–276.
- Gerischer, H. An Interpretation of the Double-Layer Capacity of Graphite-Electrodes in Relation to the Density of States at the Fermi Level. J. Phys. Chem. 1985, 89, 4249–4251.
- Randin, J.P.; Yeager, E. Effect of boron addition on the differential capacitance of stress-annealed pyrolytic graphite. J. Electroanal. Chem. 1974, 54, 93–100.
- Randin, J.P.; Yeager, E. Differential capacitance study on the edge orientation of pyrolytic graphite and glassy carbon electrodes. J. Electroanal. Chem. 1975, 58, 313–322.
- Velicky, M.; Toth, P.S.; Woods, C.R.; Novoselov, K.S.; Dryfe, R.A.W. Electrochemistry of the Basal Plane versus Edge Plane of Graphite Revisited. J. Phys. Chem. C 2019, 123, 11677–11685.
- Bauer, H.H.; Spritzer, M.S.; Elving, P.J. Double-Layer capacity at a pyrolytic graphite disk electrode. J. Electroanal. Chem. 1968, 17, 299–306.
- Krüger, A. Neue Kohlenstoffmaterialien; Teubner-Verlag: Wiesbaden, Germany, 2007.
- Kinoshita, K. Carbon: Electrochemical and Physicochemical Properties; Wiley: New York, NY, USA, 1988.
- Lobato, B.; Suarez, L.; Guardia, L.; Centeno, T.A. Capacitance and surface of carbons in supercapacitors. Carbon 2017, 122, 434–445.
- Stoeckli, F.; Centeno, T.A. Optimization of the characterization of porous carbons for supercapacitors. J. Mater. Chem. A 2013, 1, 6865–6873.
- Gagnon, E.G. Triangular voltage sweep method for determining double-layer capacity of porous-electrodes 4. Porous carbon in potassium hydroxide. J. Electrochem. Soc. 1975, 122, 521–525.
- Wen, Y.H.; Cao, G.P.; Cheng, J.; Yang, Y.S. Relationship between electrolyte ion and double-layer capacitance of carbon electrode. Acta Phys. Chim. Sin. 2005, 21, 494–498.
- Ge, Y.; Liu, Z.; Wu, Y.; Holze, R. On the utilization of supercapacitor electrode materials. Electrochim. Acta 2021, 366, 137390.
- Chmiola, J. Pore-Size Ion-Size Correlations for Carbon Supercapacitors. Ph.D. Thesis, Drexel University, Philadelphia, PA, USA, 2009.
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.L.; Gogotsi, Y.; Simon, P. Relation between the ion size and pore size for an electric double-layer capacitor. J. Am. Chem. Soc. 2008, 130, 2730.
- Feng, G.; Qiao, R.; Huang, J.; Sumpter, B.G.; Meunier, V. Ion distribution in electrified micropores and its role in the anomalous enhancement of capacitance. ACS Nano 2010, 4, 2382.
- Ania, C.O.; Pernak, J.; Stefaniak, F.; Raymundo-Piñero, E.; Béguin, F. Polarization-induced distortion of ions in the pores of carbon electrodes for electrochemical capacitors. Carbon 2009, 47, 3158.
- Chmiola, J.; Yushin, G.; Gogotsi, Y.; Portet, C.; Simon, P.; Taberna, P.L. Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 2006, 313, 1760.
- Chmiola, J.; Largeot, C.; Taberna, P.L.; Simon, P.; Gogotsi, Y. Desolvation of ions in subnanometer pores and its effect on capacitance and double-layer theory. Angew. Chem. Int. Ed. 2008, 47, 3392.
- Hsieh, C.T.; Teng, H. Influence of oxygen treatment on electric double-layer capacitance of activated carbon fabrics. Carbon 2002, 40, 667–674.
- Wu, Y.; Holze, R. Self-discharge in supercapacitors: Causes, effects and therapies: An overview. Electrochem. Energy Technol. 2021, 7, 1–37.
- Chen, X.; Wu, Y.; Holze, R. Ag(e)ing and Degradation of Supercapacitors: Causes, Mechanisms, Models and Countermeasures. Molecules 2023, 28, 5028.
- Rosen, M.J. Surfactants and Interfacial Phenomena; Wiley: New York, NY, USA, 1989.
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2012.
- Conway, B.E.; Birss, V.; Wojtowicz, J. The role and utilization of pseudocapacitance for energy storage by supercapacitors. J. Power Sources 1997, 66, 1–14.
- Conway, B.E.; Gileadi, E. Kinetic Theory of Pseudo-Capacitance and Electrode Reactions at Appreciable Surface Coverage. Trans. Faraday Soc. 1962, 58, 2493.
- Conway, B.E. Transition from “Supercapacitor” to “Battery” behavior in electrochemical energy storage. J. Electrochem. Soc. 1991, 138, 1539.
- Dubal, D.P.; Holze, R. Synthesis, properties, and performance of nanostructured metal oxides for supercapacitors. Pure Appl. Chem. 2014, 86, 611.
- Dubal, D.P.; Chodankar, N.R.; Gomez-Romero, P.; Kim, D.H. Fundamentals of Binary Metal Oxide-Based Supercapacitors. In Metal Oxides in Supercapacitors; Dubal, D.P., Gomez-Romero, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 79–98.
- Holze, R. From current peaks to waves and capacitive currents-on the origins of capacitor-like electrode behavior. J. Solid State Electr. 2017, 21, 2601–2607.
- Grahame, D.C. Properties of the Electrical Double Layer at a Mercury Surface. I. Methods of Measurement and Interpretation of Results. J. Am. Chem. Soc. 1941, 63, 1207–1215.
- Szubzda, B.; Szmaja, A.; Halama, A. Influence of structure and wettability of supercapacitor electrodes carbon materials on their electrochemical properties in water and organic solutions. Electrochim. Acta 2012, 86, 255–259.
- Trasatti, S. Progress in the Understanding of the Structure of the Metal Electrode/Solution Interface. Evolution of the Concept of Hydrophilicity. Croat. Chem. Acta 1987, 60, 357–370.
- Yoshida, A.; Tanahashi, I.; Nishino, A. Effect of concentration of surface acidic functional groups on electric double-layer properties of activated carbon fibers. Carbon 1990, 28, 611–615.
- Gu, W.; Yushin, G. Review of nanostructured carbon materials for electrochemical capacitor applications: Advantages and limitations of activated carbon, carbide-derived carbon, zeolite-templated carbon, carbon aerogels, carbon nanotubes, onion-like carbon, and graphene. Wires Energy Environ. 2014, 3, 424–473.
- Robson, R.J.; Dennis, E.A. The size, shape, and hydration of nonionic surfactant micelles. Triton X-100. J. Phys. Chem. 1977, 81, 1075–1078.
- Le, T.T.Y.; Hussain, S.; Lin, S.Y. A study on the determination of the critical micelle concentration of surfactant solutions using contact angle data. J. Mol. Liq. 2019, 294, 111582.
- Hunter, R.J. Foundations of Colloid Science; Oxford University Press: Oxford, UK, 2001.
- Bard, A.J.; Inzelt, G.; Scholz, F. Electrochemical Dictionary, 2nd ed.; Springer-Verlag: Heidelberg, Germany, 2012.
- Fang, B.; Binder, L. Enhanced surface hydrophobisation for improved performance of carbon aerogel electrochemical capacitor. Electrochim. Acta 2007, 52, 6916–6921.
- Fang, B.; Binder, L. A modified activated carbon aerogel for high-energy storage in electric double layer capacitors. J. Power Sources 2006, 163, 616–622.
- Wei, Y.Z.; Fang, B.; Iwasa, S.; Kumagai, M. A novel electrode material for electric double-layer capacitors. J. Power Sources 2005, 141, 386–391.
- He, T.; Ren, X.; Cai, K.; Wei, Y.; Sun, S. Electrochemical performance of activated carbon treated by vacuum impregnation using fluorinated surfactant. Mater. Technol. 2013, 28, 364–369.
- Fic, K.; Lota, G.; Frackowiak, E. Effect of surfactants on capacitance properties of carbon electrodes 2011, Mater. Res. Soc. Symp. Proc. 1333, doi:10.1557/opl.2011.1477.
- Fic, K.; Lota, G.; Frackowiak, E. Effect of surfactants on capacitance properties of carbon electrodes. Electrochim. Acta 2012, 60, 206-212.
- Fic, K.; Lota, G.; Frackowiak, E. Electrochemical properties of supercapacitors operating in aqueous electrolyte with surfactants. Electrochim. Acta 2010, 55, 7484-7488.
