Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Food Science & Technology
Secondary metabolites and phytochemicals in plant-based diets are known to possess properties that inhibit the development of several diseases including a variety of cancers of the aerodigestive tract. Berries are currently of high interest to researchers due to their high dietary source of phytochemicals. Black raspberries (BRB), Rubus occidentalis, are of special interest due to their rich and diverse composition of phytochemicals.
- black raspberries
- phytochemicals
- colorectal cancer
- esophageal cancer
- oral cancer
- head and neck squamous cell carcinoma
- anthocyanins
1. Black Raspberries and Cancer
1.1. Head and Neck Cancer
Most malignancies from the head and neck region are derived from the mucosal epithelium in the oral cavity, pharynx, and larynx, which are known collectively as head and neck squamous cells carcinomas (HNSCC) [20]. In the past few years, several research studies have been conducted to assess the potential of using BRB independently or in conjunction with other treatments for addressing oral cancer. In a study conducted by Chen et al., it was found that in mice exposed to dibenzo pyrene (DBP), an environmental pollutant found in polyaromatic hydrocarbons and a constituent of tobacco smoke, dietary administration of 5% significantly decreased tumor incidence (70% to 46.7%). BRB administration also resulted in a significant reduction in the levels of DBP-DNA adducts within the oral cavities of the mice [21]. Other mechanisms associated with BRB-mediated chemoprevention of HNSCC in this model include increased p120ctn expression [22]. In a different animal model of HNSCC, 7,12-dimethylbenz(a)anthracene (DMBA), induced hamster cheek pouch (HCPs), the preclinical efficacy of the topical application of BRB on chemoprevention of oral cancer was demonstrated by reduced tumor multiplicity, tumor incidence, and proliferation rate. Short-term topical delivery of BRB significantly up-regulated gene expression of retinoblastoma, a canonical tumor suppressor gene that is inactivated during HNSCC progression in high-at-risk mucosa of DMBA-induced HCPs [23]. These studies were corroborated in another HCP HNSCC model using 5% and 10% lyophilized BRB as dietary chemopreventive agents. Interestingly, 5% BRB was more effective at inhibiting HNSCC tumor development than 10% BRB. (Figure 2) These results provide support for earlier studies demonstrating the chemopreventive potential of BRB and, importantly, establish that a dietary intake of BRB can inhibit tumor formation in the oral cavity [24].
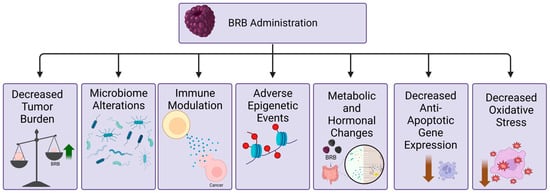
Figure 2. Schematic representation of the effects of BRB administration in cancers of the aerodigestive tract (created with BioRender.com accessed 29 December 2023). BRB-mediated decrease in tumor burden can be attributed to alterations in the microbiome, modulation of the immune response, changes in epigenetic events, metabolic and hormonal pathways, decreased expression of anti-apoptotic genes, and decreased oxidative stress.
Our group has previously demonstrated the effect of BRB on 4NQO-induced HNSCC in F344 rats. Like the HCP model, dietary administration of 5% and 10% BRB reduced the incidence and multiplicity of oral lesions by 39.3% and 28.6%, respectively. The inhibition of oral lesion by BRB was associated with reduced gene expression of anti-apoptotic and cell cycle associated markers (Aurka, Birc5, Ccna1, and Ccna2), and protein expression of Ki-67 in tongue epithelial tissues. The polyphenol ellagic acid, a phytochemical in BRB, showed similar effects [25]. Novel mechanisms of BRB chemoprevention have been identified using experimental animal models. A recent report using 4NQO-induced HNSCC in C57Bl/6 mice and F344 rats showed BRB mediated the modulation of HSD11B2, an enzyme which mediates the conversion of the active glucocorticoid cortisol into inactive cortisone. These findings demonstrated that BRB extract upregulated the HSD11B2 gene and protein expression in HNSCC cells in vitro and in vivo, resulting in a concurrent reduction in the levels of active glucocorticoids in HNSCC-induced mouse tongues (Figure 2) [26].
The immunomodulatory effect of BRB in the HNSCC tumor microenvironment has been explored in our laboratory. Our findings revealed that dietary BRB administration can inhibit the recruitment of regulatory T cells while simultaneously enhancing cytotoxic CD8 T cell activity within the tumor microenvironment. This enhanced activity was characterized by an increased production of granzyme B in the tumor site during BRB-mediated chemoprevention of HNSCC [12]. These results were further corroborated in a novel genetically modified mouse model of HNSCC involving intralingual tamoxifen injection in mouse with a conditional deletion of Tgfβr1 and Pten which resulted in HNSCC tumors that closely resembled clinical HNSCC in histology, molecular profile, and lymph node metastasis. Using this model, BRB administration over a 5-week period led to several noteworthy outcomes, including a reduction in tumor growth, an increased influx of anti-tumoral T cells into the tumor microenvironment, and the augmentation of anti-tumoral cytotoxic CD8+ T cell activity, characterized by increased expression of granzyme B and perforin (Figure 2) [27]. Collectively, the studies provide evidence for BRB-mediated promotion of anti-tumoral immune responses, mediated by improved anti-tumoral T cell infiltration, which opens the potential of combinatorial approaches with checkpoint inhibitors for improved HNSCC treatment outcomes.
Several studies have reported on the potential efficacy of BRB formulations in the chemoprevention of HNSCC in clinical patients. A 6-week trial was performed utilizing a mucoadhesive gel formulation of 10% freeze-dried BRB [28,29,30]. Patients with oral pre malignancies applied the gel four times daily, delivering 0.5 g of BRB in each application. Patients were biopsied before and after the 6-week trial period, along with normal tissue for comparison [29]. No patients within the study reported adverse events from the gel application. BRB gel treatment resulted in histological regression in a subset of patients and a statistically significant reduction in the loss of heterozygosity [29]. This study was further expanded in the same cohort with the goal to explore alteration in growth factors, proinflammatory and angiogenic enzymes, gene expression profiles, and micro vessel density [31]. The BRB gel showed greater suppression of genes associated with RNA processing, growth factor recycling, and inhibition of apoptosis. Furthermore, COX-2 levels were reduced along with micro vessel density [31]. These studies are very informative on potential translational applications of BRB formulations in HNSCC chemoprevention. Interestingly, the authors noted that a subpopulation of patients appeared more responsive to the treatment [31]. Underlying biological and molecular mechanisms that distinguish responders from non-responders to BRB chemoprevention of HNSCC will be crucial in determining clinical application. Further expansion of this study to a multi-centered placebo-controlled trial involving forty patients over a 3-month period [28] was performed. A total of 22 patients received the 10% BRB gel treatment, while 18 patients received the placebo gel. Application of the gels occurred four times daily with a total of 0.5 g of BRB being administered with each application to the oral premalignant lesion. The BRB group saw significant reductions in lesion size, histological grade, and loss of heterozygosity compared to the placebo group [28]. However, variation in responsiveness to the BRB treatment was observed among patients, as measured by the different levels of BRB metabolic and keratinocyte differentiation enzymes in patient lesions [28]. In another clinical trial, patients with confirmed oral squamous cell carcinomas (OSCC) were administered three dissolvable slow-release oral troches daily containing 4.3 g freeze-dried BRB powder until resection surgery [32]. Following BRB administration, pro-survival genes AURKA, BIRC5, and EGFR and proinflammatory genes NFKB1 and PTGS2 were significantly reduced. Furthermore, the phytochemicals cyanidin-3-rutinoside and cyanidin-3-xylosylrutinoside were detected in all OSCC patient tissues (Figure 2) [32]. These studies demonstrate the ability of BRB to modulate the transcriptional profile of HNSCC cells in a manner that supports a chemopreventive role, and builds on the findings that local delivery of BRB treatment to the target site results in better and more consistent absorption in HNSCC patients, which suggests an effective strategy for management of these cancers [33].
1.2. Esophageal Cancer
Esophageal cancers (EC) are derived from the cells that line the inside of the esophagus, with most being adenocarcinomas and squamous cell carcinomas [34]. Its subtle early symptoms and aggressive nature make it a challenging cancer to treat, with only a 15–20% five-year survival rate [35]. As there is an ongoing search for innovative ways to prevent and treat EC, the potential role of BRBs has garnered attention for its promising properties [36]. In a recent study by Shi et al., N-nitrosomethylbenzylamine (NMBA)-induced rats were used to assess the effect of BRB on oxidative stress and its related oncogenic signaling pathways such as the NF-kB/MAPK to further understand the underlying mechanisms of the anti-cancer action of BRB. Tumor incidence and tumor multiplicity in rats fed 5% BRB was significantly reduced as compared to those fed a control diet. In addition, the mRNA expression of SOD2 (an essential antioxidant defense against oxidative stress) and GPx (glutathione peroxidase, an antioxidant enzyme that scavenges free radicals to prevent lipid peroxidation as well as maintain redox balance to prevent damage to DNA, proteins, and lipid membranes) [37,38], were assessed. While SOD2 and Gpx expression were decreased in NMBA-induced rats leading to oxidative stress, NMBA-induced rats fed a BRB diet demonstrated increased SOD2 and GPx gene expression. BRB was also observed to significantly suppress the phosphorylation of the oncogenic signaling NF-κB and MAPK pathways. Overall, this study showed that BRB contributes to the alleviation of EC incidence as well as tumor multiplicity by reversing oxidative stress and suppressing NF-kB/MAPK pathways, suggesting that this could be a potential mechanism of EC chemoprevention by BRB (Figure 2) [39]. Similar results were found in other studies evaluating the effects of dietary BRB on experimentally induced EC, with additional mechanisms associated with pro-inflammatory cytokines modulation (including CCL2, S100A8, and IL-19), prevention of aberrant DNA methylation, which is a common occurrence in the development of EC [40], and reduced gene expression of DNA methyltransferases, Dnmt1 and Dnmt3b, in both dysplastic lesions and in papilloma of the esophagus [41].
The bioactive phytochemicals in BRB have been further explored in EC chemoprevention [42]. Peiffer et al. explored the role of BRB and its component anthocyanins (AC) and protocatechuic acid (PCA) in inhibiting the development of EC in rats induced by NMBA. Rats were fed with diets containing 6.1% BRB powder, an AC-rich fraction of BRBs (3.8 μmol/g), or 500 ppm PCA. All three diets had similar effects on cytokine production in the plasma and esophagus with decreased production of pro-inflammatory cytokine IL1β and increased expression of the anti-inflammatory cytokine IL-10. An increased expression of IL-12, which is known to activate cytotoxic NK cells and CD8+ T cells, was also observed in all dietary administrations. Downregulation of pro-inflammatory cytokines, upregulation of anti-inflammatory cytokines and alteration of the immune cell infiltration into tumor tissues were demonstrated to be mechanisms associated with BRB-, AC-, and PCA-mediated chemoprevention of EC (Figure 2) [43].
In addition to animal studies that employed NMBA-induced rat models, a few in vitro studies have explored the mechanistic role of BRB as well as its individual phytochemical constituents in the prevention of esophageal carcinogenesis. One such study evaluated an ethanol (EtOH) extract of BRB and the two component anthocyanins (cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside) in BRB for their effects on growth, apoptosis, and gene expression in rat esophageal epithelial cell lines RE-149 and RE-149 DHD. A dose-dependent growth inhibition of RE-149 DHD cells by BRB extract was observed. An amount of 100 µg/mL was the most effective inhibitory concentration of the extract, resulting in a 45% and 16% inhibition in the growth of RE-149 DHD and RE-149 cells, respectively. However, the growth inhibition was significant only for the RE-149 DHD cells. Similar to BRB extract, cyanidin-3-O-glucoside and cyanidin-3-O-rutinoside, were seen to selectively cause significant growth inhibition and induction of apoptosis in a highly tumorigenic cell line RE-149 DHD but not in a weakly tumorigenic line RE-149. Mechanistically, increased Caspase 3 and Caspase 7 expression were associated with effects of BRB and two component anthocyanins on treated cells. However, a significant reduction in COX-2 and i-NOS expression was observed only with cyanidin-3-O-glucoside or cyanidin-3-O-rutinoside but not BRB extract. Taken together, this study revealed that the EtOH BRB extract and two component anthocyanins inhibit the growth and apoptosis of highly tumorigenic rat esophageal epithelial cells in vitro by affecting the gene expressions of Caspase 3 and 7 as well as COX-2 and i-NOS (Figure 2) [44]. Other associated mechanisms of BRB phytochemical-mediated inhibition of EC as revealed by in vitro studies include the suppression of human β-defensin 2 (HBD-2), a protein expressed in the esophagus in response to stress or infectious agents that possesses an oncogenic role in the initiation and progression of esophageal SCC. These effects were observed in cyanidin-3-glucoside and cyanidin-3-rutinoside treatment of the esophageal SCC cell line KYSE-150, in a dose-dependent manner, which was similar to treatment with cancer drugs PBIT and celecoxib [45].
1.3. Colorectal Cancer
Colorectal cancers (CRC) originate from cells in the inner lining of the colon or rectum that start off as growths known as polyps [51]. Most tend to be adenocarcinomas derived from mucus-producing cells [51]. In vitro studies demonstrate an important role for BRBs in inhibiting CRC growth, and several mechanisms have been identified. One such study using human CRC cell lines SW480 and Caco2, demonstrated a growth inhibitory effect of a BRB extract, with mechanisms associated with the promotion of histone acetylation, upregulation of p65, and downregulation of IκB, pathways which are known to be important in CRC progression [52,53,54]. Similar inhibitory effects of BRB on cancer cell proliferation, migration, and colony formation in vitro were observed in HCT-116 and LoVo CRC cell lines [55].
In vivo studies using a mouse model of CRC with azoxymethane (AOM-induced CRC model) corroborates these in vitro results [56]. This study demonstrated that a BRB diet (4.1 g/kg) significantly reduced tumor multiplicity, emphasizing BRB’s role in preventing the progression of CRC. In line with previous in vitro experiments, these in vivo studies demonstrate BRB-mediated reduction in the anti-apoptotic protein Bcl-2 and increased expression of the apoptotic protein, Bax (Figure 2) [53]. Similarly, Chen et al. observed a significant reduction in tumor multiplicity in a chemically induced CRC (AOM/DSS) mouse model after dietary administration of 5% and 10% BRB [55].
BRB has also been shown to modulate antitumor immunity in CRC. Innate immune cells such as natural killer (NK) cells play a significant role in mitigating CRC development and progression. A recent study investigated underlying mechanisms of NK cell effects on CRC progression, and the potential role BRB extract plays in modulating this innate immune population during CRC chemoprevention [57]. BRB was shown to enhance NK cell infiltration and suppress CRC development. Studies on clinical CRC patients further demonstrate the efficacy of BRB phytochemicals in enhancing NK cell cytotoxicity [58]. Further evidence for BRB-mediated immunomodulation in CRC is demonstrated by other studies investigating the potential of BRBs to modulate inflammation and cytokine production during CRC [59]. Growing evidence has shown that inflammation and cytokines can promote carcinogenesis including processes in the development of CRC [60]. Twenty-four patients with CRC, who had not received prior treatment, consumed 20 g of freeze-dried BRB powder three times daily for three weeks until surgical resection. Plasma concentration of granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-8 were significantly reduced with the BRB diet which was associated with the induction of apoptosis and reduced proliferation in colorectal tissues [59].
Epigenetic modification of tumor suppressor genes has been found to be an associated mechanism of BRB chemoprevention in clinical CRC patients. Wang et al. sought to evaluate the effects BRBs have on biomarkers of tumor development in human CRC, specifically focusing on methylation patterns of relevant tumor suppressor genes, cell proliferation, apoptosis, angiogenesis, and Wnt pathway genes [61]. In this study of 20 enrolled patients, BRB powder was administered orally (60 g/day for 1 to 9 weeks), a concentration equivalent to a rodent diet of about 7% BRB powder, which was confirmed to have chemopreventive properties in the rat colon [14,62]. Patients enrolled in this study were not currently receiving chemotherapy or radiation therapy. Immunohistochemical data from biomarkers Ki-67, TUNEL, β-catenin, CD105, and DNMT1 indicated that they were modulated protectively in the tissues of all patients. However, positive DNA methylation modulation only occurred in patients that were treated with BRBs for an average of 4 weeks, suggesting that BRB treatment required a relatively long period of administration to be effective [61]. Studies have shown that DNA methyltransferase (DNMTs) are overly expressed in several tumor types including CRC, and knockout experiments with cell lines have demonstrated increased expression of tumor suppressor genes such as p16 (Figure 2) [63,64]. Therefore, inhibition of DNMTs may be a promising target for chemoprevention of CRC [65]. This study provided evidence of the ability of BRBs to demethylate tumor suppressor genes and to modulate other biomarkers of tumor development in human CRC.
Metabolomic analysis has further revealed additional mechanisms associated with BRB-mediated chemoprevention of CRC. One such study by Pan, P. et al. [66] investigated BRB-mediated metabolite changes from 28 CRC patients who were given 60 g of BRB powder daily for 1 to 9 weeks, following the same administration protocol as Wang et al. [61]. Patients in this study were also not currently receiving chemotherapy or radiation therapy. Non-targeted metabolic analysis uncovered over 400 annotated metabolites, with 34 and 16 metabolites significantly changed by BRB in the urine and plasma, respectively. Increased levels of 4-methylcatechol sulfate were observed in both post-BRB urine and post-BRB plasma, which were correlated with higher levels of apoptosis in post-BRB tumors. Furthermore, polyphenols derived from BRB were absorbed and metabolized to various benzoate species, which were found to be increased in post-BRB patients. These were correlated with enhanced levels of amino acid metabolites [66]. This study suggests BRBs induce significant metabolic changes in CRC patients, and BRB-mediated regulation of metabolic pathways may be beneficial against CRC.
2. Black Raspberries and Other Diseases
2.1. BRBs and Allergic Inflammation
Allergic inflammation arises from a distinct set of cellular and humoral reactions, culminating in the initiation of both the innate and adaptive immune systems. The dietary impacts of BRB have been observed in allergic conditions such as contact hypersensitivity (CHS). Our in vivo findings indicate that dietary administration of BRB extract and its anthocyanin metabolite, protocatechuic acid (PCA), can alleviate the pathology of contact hypersensitivity [74]. Mice on BRB- or PCA-supplemented diets had reduced contact allergen-induced ear swelling and less accumulation of activated DCs in the spleen after exposure to di-nitro fluorobenzene (DNFB). In vitro, BRB extract decreased DC maturation, Cd80 expression, and IL-12 secretion, while PCA reduced IL-12 levels. Dietary BRB and PCA led to varying decreases in IL-12-related CHS-mediated effector mechanisms, including IFN-γ, IL-2, and IL-17 production by T cells [74,75]. BRB fractions and a mixture of these cyanidins present in BRBs were effective in reducing lipopolysaccharide (LPS)-induced iNOS expression and other inflammatory markers such as tumor necrosis factor (TNF)-α, IL-6, and IL-1β. These effects were achieved by inhibiting the phosphorylation of mitogen-activated protein kinases (MAPKs) and STAT3 in murine macrophage RAW264.7 cells [76]. The anti-inflammatory properties of BRB phytochemicals contribute to their wide application in mitigating the pathology of diseases characterized by chronic inflammation.
2.2. BRBs and Cardiovascular Disease
BRBs have gained recognition for promoting cardiovascular well-being, evidenced by their capacity to lower levels of blood trimethylamine-N-oxide (TMAO), a metabolite produced by gut bacteria [77]. This metabolite has been linked to cardiovascular disease and was investigated in a study conducted using Sprague-Dawley rats, which were subjected to a high-fat diet supplemented with TMAO. Notably, dietary intake of TMAO resulted in elevated serum LDL cholesterol, whereas the consumption of BRB extract contributed to a reduction in this cholesterol biomarker [77]. In a separate study, it was demonstrated that BRB extract possesses the capability to induce alterations in the gut bacterial community. Additionally, BRB can modulate bile acids and regulate gene expression patterns, all of which collectively contribute to the potential of reducing cholesterol levels [78]. An investigation focusing on vascular senescence, a process associated with the advancement of cardiovascular diseases, revealed that polyphenol extracts from BRB displayed efficacy in diminishing Angiotensin II-induced senescence. This effect was achieved by increasing the cellular antioxidant capabilities, notably by elevating the expression of key antioxidant enzymes, including superoxide dismutase (SOD) 1, SOD2, and glutathione peroxidase [79].
BRB extract was utilized to investigate its potential anti-inflammatory and metabolic modulatory effects during choline-induced inflammation on rats fed a high-fat diet [80]. Various epidemiological studies have revealed a connection between choline and cardiovascular diseases, diabetes, and renal diseases, specifically focusing on vascular inflammation, endothelial dysfunction, and cholesterol homeostasis [81,82,83,84,85,86,87]. The study showed that a consistent intake of BRB extract was able to lower the levels of trimethylamine-N-oxide (TMAO) cecal and trimethylamine (TMA) serum, products of metabolized choline, which indicates an improvement in the serum lipid profile in diet-induced hypercholesterolemia in rats [88]. This suggests that BRBs may act as a prebiotic in the human gut and an agent to alleviate hepatic inflammation [80]. However, as the authors noted, further studies including microbiome analysis are needed to understand these observations.
2.3. BRBs and Hepatic Inflammation
Alcohol use disorder (AUD) is a significant public health concern on a global scale [89]. Excessive and long-term consumption of alcohol can lead to adverse effects in the digestive, urinary, and circulatory system [90]. Alcohol abuse may lead to alcoholic liver disorders (ALD) which include alcoholic hepatitis, fatty liver, liver fibrosis, and cirrhosis [91,92]. Anthocyanins from BRBs have been tested to determine effects on protection against alcohol liver diseases and hepatic inflammation. Xiao T et al. demonstrated the potential preventative role of BRB on ALD. Histopathological observations in sub-acute ALD mice administered BRBs displayed reversal of liver damage. Additional evidence for regulation of inflammation was found through decreased expression levels of NF-kb and TGF-b after treatment with BRBs in both acute and subacute treatment groups. The results demonstrate that BRBs possess preventative effects on ALD [93].
2.4. BRBs and Myelodysplastic Syndromes
BRBs have been used in a pilot clinical study to investigate their potential to treat myelodysplastic syndromes (MDS) and myelodysplastic/myeloproliferative neoplasms (MDS/MPN). These are bone marrow disorders characterized by cytopenia and eventual progression into acute myeloid leukemias [94]. Stem cell transplantation remains the only cure, but transfusions and growth factor therapies are also used for low-risk MDS [95]. Two FDA-approved drugs, azacytidine and decitabine, are used in high-risk MDS patients who can tolerate them; however, these drugs are associated with significant cytopenia and gastrointestinal toxicity [96]. A group of researchers who previously demonstrated the hypomethylating effects of BRBs in patients with colon cancer and familial adenomatous polyposis explored if BRB displayed similar hypomethylating effects as in conventional MDS treatment agents [61,72]. The trial demonstrated that a daily dose of 50 g of BRB powder mixed in water taken over the course of three months by patients with MDS and MDS/MPN lead to regulation of leukocytes and T-cell differentiation along with increased white blood cell counts. These observations were attributed to both a hypomethylation of intergenic regions and a hypermethylation of intragenic and intergenic regions. These effects also manifested without significant side effects or adverse effects in study patients which demonstrates the desired safety profile of BRB intervention [97]. The precise impact of the observed hypermethylation events will require further investigation.
2.5. BRBs and the Microbiome
The microbiome plays an important role in nutrient breakdown and synthesis, providing an antimicrobial barrier against potential pathogens, providing signals for immunomodulation, and assisting in the retention of structural components where they reside [98,99]. Within the gastrointestinal tract, there is mounting evidence demonstrating the integral role that a healthy microbiome plays in the prevention and detection of several diseases [100,101]. Major aberrations within the gastrointestinal microbiome have been associated with luminal diseases like inflammatory bowel disease (IBD) and irritable bowel syndrome, metabolic diseases like obesity and diabetes, allergic conditions, and neurodevelopmental illnesses [102,103,104,105]. Evidently, the microbiome plays a major role in the maintenance of healthy tissue and affects disease progression and prognosis [106,107]. Even as an understanding of relations between disease outcomes and gut microbial patterns have begun to emerge, the associations between host and gut bacteria metabolites are not well documented. Specialized metabolites within the gut microbiome have now been demonstrated as contributing to alterations in normal host physiology [105,108,109]. Metabolic products from bacteria which bind to the aryl hydrocarbon receptor (AHR) have been shown to affect immune cells and the mucosal lining [110,111]. Conditions such as Crohn’s disease and ulcerative colitis involve a complex disarray of inflammation within the digestive tract from abnormal gut bacteria and host immune responses. AHR expression has been found to be diminished within individuals suffering from IBD; however, diets supplemented with BRB increased fecal AHR activity in experimental studies [112,113]. These results display the potential that a diet supplemented with BRB may have on the modulation of key metabolites reducing intestinal inflammation associated with IBD. BRB phytochemicals and other constituents such as vitamins, calcium, and fiber [16] are metabolized by host microbes, generating secondary metabolites that contain antioxidant, antiproliferative, and pro apoptotic properties [114,115]. It has been shown that BRBs are able to promote the production of short-chain fatty acids utilizing gut microbe fermentation [116]. Furthermore, BRB consumption has been associated with the promotion of beneficial gut bacteria growth, like Akkermansia muciniphila, Bifidobacterium species, and Lactobacillus species, while inhibiting the growth of pathogenic strains and species such as Helicobacter pylori (Figure 2) [55,117]. These microbiome changes are beneficial for the host to prevent the development of inflammatory diseases and possibly cancers of the GI tract.
2.6. BRBs and Human Papilloma Virus Infection
As the most common sexually transmitted disease worldwide [118,119], management of HPV infection remains a significant public health concern. While most infections from HPV are cleared by the immune system in under two years of initial infection, a small fraction persists to preneoplastic lesions and result in cancers [120,121]. HPV infections are closely linked to cervical cancer but are also noted in a significant portion of anogenital and oropharyngeal cancer [122,123,124]. In cervical cancer, the fourth deadliest cancer in women, HPV infection has been found in over 90% of cases [125,126]. With the advent of multiple HPV vaccines becoming available, the incidence of cervical cancer, neoplasia, and genital warts has markedly reduced [127,128,129]. It is of interest that BRB phytochemicals display anti-proliferative activity against human cervical cancer cell lines [130]. Antiviral activity of BRB constituents and phytochemicals have been demonstrated in influenza A, Aichi viruses, and norovirus [131,132,133,134,135]. BRBs are currently being tested in a clinical study investigating the potential for application of BRB and lactoglobulin in the treatment of HPV and prevention of cervical cancer. In the test group, patients were administered SanuGene Vaginal Gel, composed of BRB extract, lactoglobulin, and other components within a gel matrix. With a sample of 300 outpatients divided into 200 tests and 100 control subjects, participants were given a vaginal preparation of gel every other day containing either 3 g BRB extract (test group) or placebo (control group). Preliminary results from the Tianjin Medical University report that within the test group, 72.8% of subjects had an effective clearance of HPV infection, whereas only 15.8% of subjects in the control group cleared their infection. Additionally, the HPV viral load within the test group decreased by 60%, whereas the control group increased by 21.8%. No serious side effects were reported. BRB extract in combinatorial treatment approaches, including vaginal gels, has strong implications and potential for the treatment of HPV infection and prevention of cervical cancers [136].
This entry is adapted from the peer-reviewed paper 10.3390/ph17010084
This entry is offline, you can click here to edit this entry!
