1. New Labeling Rules for Wine
The new Regulation (EU) 2021/2117
[1], published on 2 December 2021, amends the labeling rules for wines and aromatized wines, requiring the provision of all product data as defined in the food information regulation (EU) 1169/2011
[2]. From a practical standpoint, the primary objective of the new European wine labeling regulation is to ensure food safety for consumers and to secure their right to information on critical matters such as ingredient lists and nutritional tables. This regulation also aims to enhance transparency within the sector, fostering greater consumer trust. This new European regulation came into force on 8 December 2023.
Nevertheless, under specific conditions, it is permissible to provide consumers with this information through electronic labeling or e-label. If the operator wishes to indicate the ingredient list on an electronic label, they should nonetheless disclose the presence of allergenic substances on the physical label. If nutritional information is provided on the electronic label, the operator must indicate the energy value of 100 mL of wine or flavored wine on the physical bottle label (the option to use the energy symbol “E”). Alternatively, the operator may choose to display the ingredients and nutritional declaration on the packaging or on an attached label. The regulation provides for a transition period of 2 years. All wines produced and labeled after 8 December 2023 will be required to include the ingredient list and nutritional information.
In accordance with the consumer information regulation (EU) 1169/2011, the definition of an ingredient is any substance or product, including flavorings, food additives, and food enzymes, or any constituent of a compound ingredient used in the manufacture or preparation of a food product and remains present in the finished product, possibly in a modified form; residues are not considered ingredients. In this regard, Regulation (EU) 2019/934
[3], dated 12 March 2019, lists authorized enological practices, along with their classification, as additives or technological auxiliaries. Only additives are subject to labeling. The new labeling must indicate the category of the wine product and, if applicable, whether it is “dealcoholized” or “partly dealcoholized” wine. Furthermore, references to PDO or PGI, alcohol content, bottler or producer name, importer (if any), sugar content in sparkling wines, allergens, nutritional information, and the ingredient list should be included. All this information must be presented in a language understandable to consumers in the country where the wine is marketed, and the characters must be of a size equal to or greater than 1.2 mm.
Regarding nutritional information, the bottles must include both the energy value (in kJ and kcal) and the amounts of fats, saturated fats, carbohydrates, sugars, proteins, and salt. All of this should be expressed per 100 g or 100 mL and should appear within the same visual field of the label, using clear typography. While the energy value is compulsory on the physical label, the rest of the nutritional information may be provided via electronic means indicated on the packaging. Nutritional values are average values based on the manufacturer’s analysis, known average values, or generally established data. If the information is provided electronically, certain additional regulations must be met. User data cannot be collected or tracked, and a web address cannot be printed on the label. All wine ingredients must be listed in decreasing order of weight, and allergens, additives, and other specific elements have their own nomenclature and regulation.
This regulation is aimed at providing greater transparency to the wine industry and, above all, at better informing consumers. In a world increasingly concerned with healthy eating and transparency in the products they consume, this change was necessary and welcomed. The main challenge of this new regulation will be its implementation, as all wineries and wine producers will need to adapt their labeling and production processes to comply with the new regulations. However, while it represents a challenge, it is also an opportunity to stand out in the market and demonstrate commitment to consumer transparency and health.
The new European Regulation (EU) 2021/2117 will transform the wine industry and provide consumers with a better understanding of what they are consuming, which will be beneficial for all. The implementation of the new labeling regulations carries important implications for consumers’ understanding of the precise meaning of each component and ingredient, as well as the associated nutritional information. In the interest of providing more detailed disclosure, this article aims to enhance consumers’ understanding of nutritional analyses, providing them with a more comprehensive perspective of the elements present in the final product. By detailing the presence and function of additives more precisely and by presenting relevant nutritional information, the goal is to empower consumers to make informed decisions about their wine consumption, aligning it with their personal preferences and dietary needs. Furthermore, wines from different geographical areas were analyzed to assess whether geographical origin significantly influences the composition of wine and the calorie intake to better understand the impact of the nutritional facts of the new labeling.
2. Spanish Regulatory Wine Classification, Winemaking Procedure, and Common Additives in Wine
In European wine law, wines are classified into several categories, each indicating different levels of quality and geographical specificity. Table wines, known as “Vino de Mesa” in Spain, are the most basic category, characterized by their simplicity and lack of specific requirements. Land wine, referred to as “Vino de la Tierra” in Spain, denotes a specific geographical region and may offer slightly higher quality than table wines. Protected Geographical Indication (PGI) wines, such as France’s “Vin de Pays” or Italy’s IGT wines, are associated with specific regions and must adhere to strict production criteria, offering consumers an assurance of quality and provenance. Finally, wines labeled as Denominación de Origen (DO), Denominazione di Origine Controllata (DOC), Denominazione di Origine Controllata e Garantita (DOCG) in Italy, or Appellation d’Origine Contrôlée (AOC) in France are considered of the highest quality and are rigorously regulated in terms of grape variety, production methods, and geographical origin, providing consumers with a guarantee of excellence and adherence to strict standards. Each category represents an incrementally higher level of quality and geographic specificity, offering valuable information about the wine’s origins and characteristics.
The winemaking process comprises several critical steps. (i) Harvesting: the grapes are carefully harvested at the optimal time, ensuring the desired level of ripeness and sugar content. (ii) Crushing and pressing: The harvested grapes are crushed to extract their juice. Typically, white wines are promptly pressed, whereas red wines may undergo maceration with the grape skins to extract color and tannins. (iii) Fermentation: Yeast is introduced to the grape juice to initiate the fermentation process, converting sugars into alcohol. This transformation can occur in stainless steel tanks, oak barrels, or other containers. (iv) Aging: Following fermentation, the wine may undergo aging in oak barrels or stainless-steel tanks to enhance its flavor and character. The duration of this aging process varies according to the desired wine style. (v) Clarification and stabilization: to achieve microbiological stability and remove remaining solids, the wine undergoes processes such as fining, filtering, and cold stabilization. (vi) Bottling: Once the wine has been clarified and stabilized, it is bottled and sealed. At this stage, winemakers may opt to incorporate specific additives, such as sulfur dioxide, to aid in wine preservation and prevent spoilage.
It is important to note that the use of additives in winemaking is carefully regulated, and winemakers must adhere to specific guidelines regarding which additives are permitted and in what quantities. These additives are used judiciously to maintain the quality and stability of the wine. Common additives in winemaking include the following: (i) sulfur dioxide: This is used as a preservative to inhibit the growth of undesirable microorganisms and to prevent oxidation. The maximum allowable levels of sulfur dioxide in wine are regulated by various government authorities, such as the US FDA in the United States and the EU regulations in Europe. (ii) Yeast nutrients: These are used to support healthy fermentation by providing essential nutrients for yeast metabolism. The specific types and maximum doses of yeast nutrients may vary depending on the winemaking practices and regulations in different regions. (iii) Fining agents: These are used to clarify the wine by removing particulate matter. Examples of fining agents include bentonite, egg whites, and various types of proteins. The types and maximum allowable doses of fining agents are typically regulated by wine authorities to ensure the safety and quality of the final product. It is important to note that regulations regarding additives in wine can vary by country and region, and winemakers must comply with specific guidelines set forth by their local regulatory bodies. Additionally, winemakers often follow industry best practices and standards to ensure that the use of additives is carefully monitored and remains within acceptable limits. For specific information on the maximum allowable doses of additives in wine, the authors recommend referring to the official regulatory agencies governing winemaking practices in your specific region. Additionally, consulting with a qualified enologist or regulatory expert can provide detailed insights into the specific regulations and guidelines applicable to winemaking and the use of additives.
Considerations of caloric intake become particularly important when consuming wine, especially for individuals managing conditions such as metabolic syndrome. This is attributed to the potential impact on overall caloric intake and metabolic health. Notably, wine, like many other alcoholic beverages, contains calories primarily from alcohol, with sweet wines also contributing calories from residual sugars. In the context of metabolic syndrome, which encompasses a cluster of conditions including high blood sugar, excess abdominal fat, abnormal cholesterol or triglyceride levels, and high blood pressure, vigilance in monitoring caloric intake is paramount. Excessive calorie consumption, particularly from alcohol, can contribute to weight gain and exacerbate metabolic syndrome. From a scientific perspective, the caloric content of wine, approximately 7 calories per gram of alcohol, plays a significant role in overall energy intake. Excessive consumption can disturb the balance between energy expenditure and intake, thus potentially contributing to weight gain and metabolic dysregulation.
Following the implementation of the new wine labeling regulations, which include the disclosure of the caloric content in a serving of wine, consumers will be empowered to comprehend the significance of understanding the caloric load of a standard glass of wine in the context of their total daily caloric intake. These data hold relevance for individuals managing conditions such as metabolic syndrome, as it equips them to make informed dietary decisions, including those related to wine consumption, in consideration of their specific health conditions. For individuals with metabolic syndrome, meticulous attention to the caloric content of wine and other dietary sources is vital to uphold a meticulously balanced and health-conscious diet. Modulation of alcohol consumption and overall caloric intake, in conjunction with an emphasis on nutrient-dense dietary options, is frequently advised for individuals managing metabolic syndrome. It is important to note that the influence of wine consumption on metabolic health is influenced by individual factors such as comprehensive dietary patterns, levels of physical activity, and any co-existing medical conditions. Seeking guidance from a healthcare professional or a qualified dietitian can facilitate tailored recommendations for navigating dietary choices, encompassing the consumption of wine, in the context of managing metabolic syndrome.
3. Grape Polyphenol Synthesis, Structure, and Composition
Two main pathways are implicated in the biosynthesis of phenolic compounds: shikimic acid and malonic acid
[4]. The malonic acid pathway is considered one of the most important sources of phenols in fungi and bacteria but is less extensively used in superior plants. On the other hand, the shikimic acid pathway is responsible for the biosynthesis of most polyphenolic compounds in plants. Starting from erythrose-4-phosphate and phosphoenolpyruvic acid, a sequence of reactions is initiated, leading to the generation of shikimic acid and several aromatic amino acids (phenylalanine, tryptophan, and tyrosine). Most polyphenolic compounds are derived from phenylalanine. Phenolic compounds are important contributors to the antioxidant properties and the color and mouthfeel of red wine
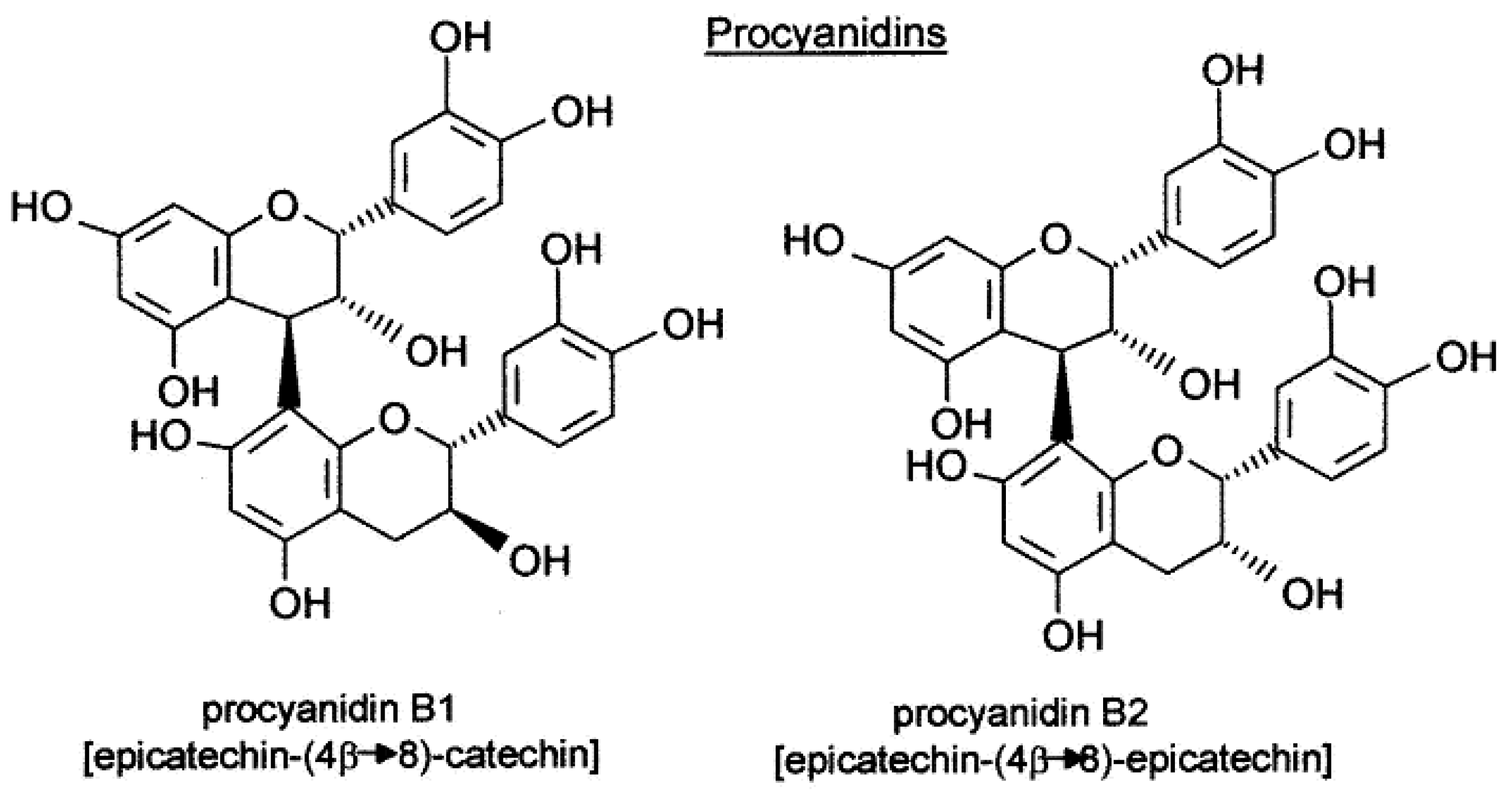
[5]. Two important families of polyphenol compounds present in grapes are known to influence final wine quality, specifically, proanthocyanins (condensed tannins) (
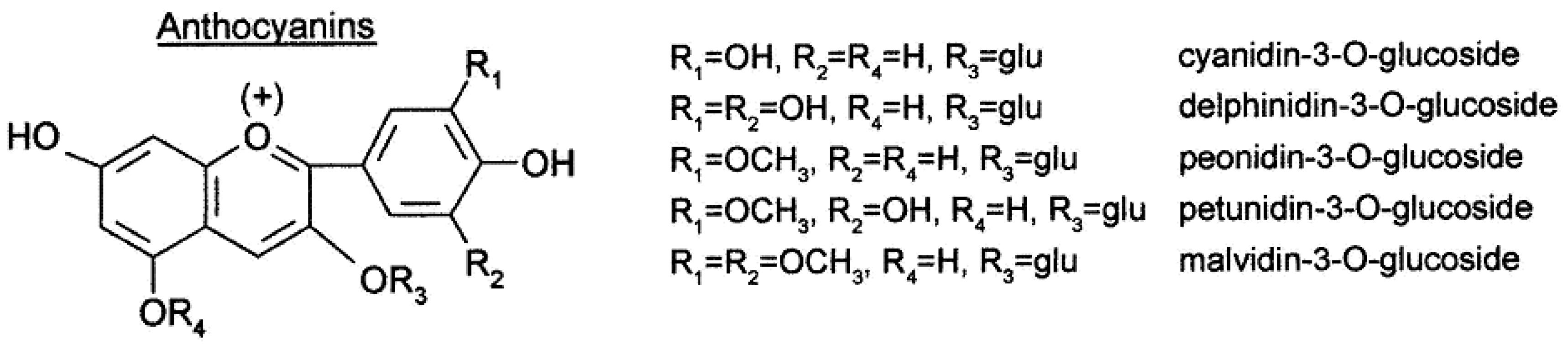
Figure 1) and anthocyanins (
Figure 2). The former contribute to the astringency and bitterness of wines, while the latter are pigments responsible for wine color
[6][7]. Polyphenol composition is attributed not only to the type of cultivar but also to the location of grapes, environmental and management practices, and the growing season
[8][9][10]. Proanthocyanins and anthocyanins constitute the two most abundant classes of phenolic compounds in berry skin. Condensed tannins are polymeric flavan-3-ols, mainly comprising subunits of (-)-epicatechin, in addition to significant amounts of epigallocatechin, (+)-catechin, and epicatechin-3-O-gallate
[11].
Figure 1. Procyanidin structure. The different subunits are linked by C4–C8 and, to a lesser extent, C4–C6 inter flavan bonds.
Figure 2. Anthocyanin structure.
Anthocyanins are responsible for the color of red and black varieties of grapes. Most Vitis Vinifera varieties produce non-acylated glucoside, acetyl glucoside, coumaroyl glucoside (and, to a lesser extent, caffeoyl glucoside) derivatives of delphinidin, cyanidin, petunidin, peonidin, and malvidin. Each variety of grape has a specific anthocyanin profile. Anthocyanin analysis has been proposed for varietal authentication of grapes and wines. Both anthocyanins and tannins are partially extracted from grape skin during winemaking and undergo structural transformations through several reactions with significant influence on wine sensory characteristics due to their involvement in astringency, bitterness, color intensity, and color stability
[11][12].
Anthocyanins represent the largest group of water-soluble pigments in the plant kingdom. These compounds are widely distributed in crops, beans, fruits, vegetables, and red wine, resulting in the human ingestion of significant amounts of anthocyanins from plant-based daily diets. In general, anthocyanin pigments are stable under acidic conditions but are unstable and rapidly broken down under neutral conditions.
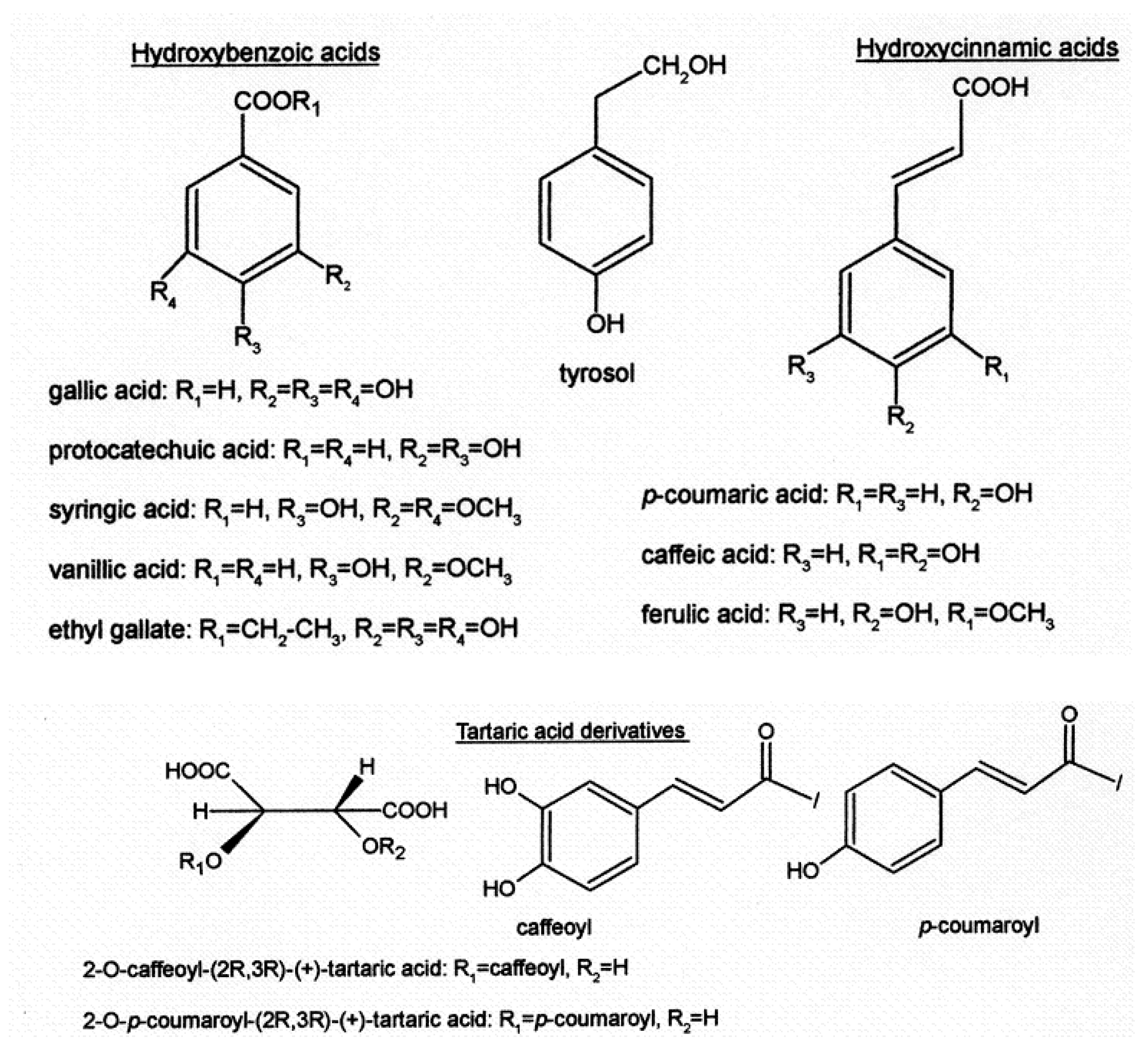
Focusing on phenolic acids (Figure 3), they are predominantly colorless molecules present in red wines. Their presence does not contribute specific aroma or flavor to the wine; however, they play an important role in the chromatic evolution of wine, potentially contributing to the yellowish tone of older red wines if oxidation occurs.
Figure 3. The main phenolic acids are also found in wine: hydroxybenzoic acid, tyrosol, hydroxycinnamic acid, tartaric acid, and derivatives.
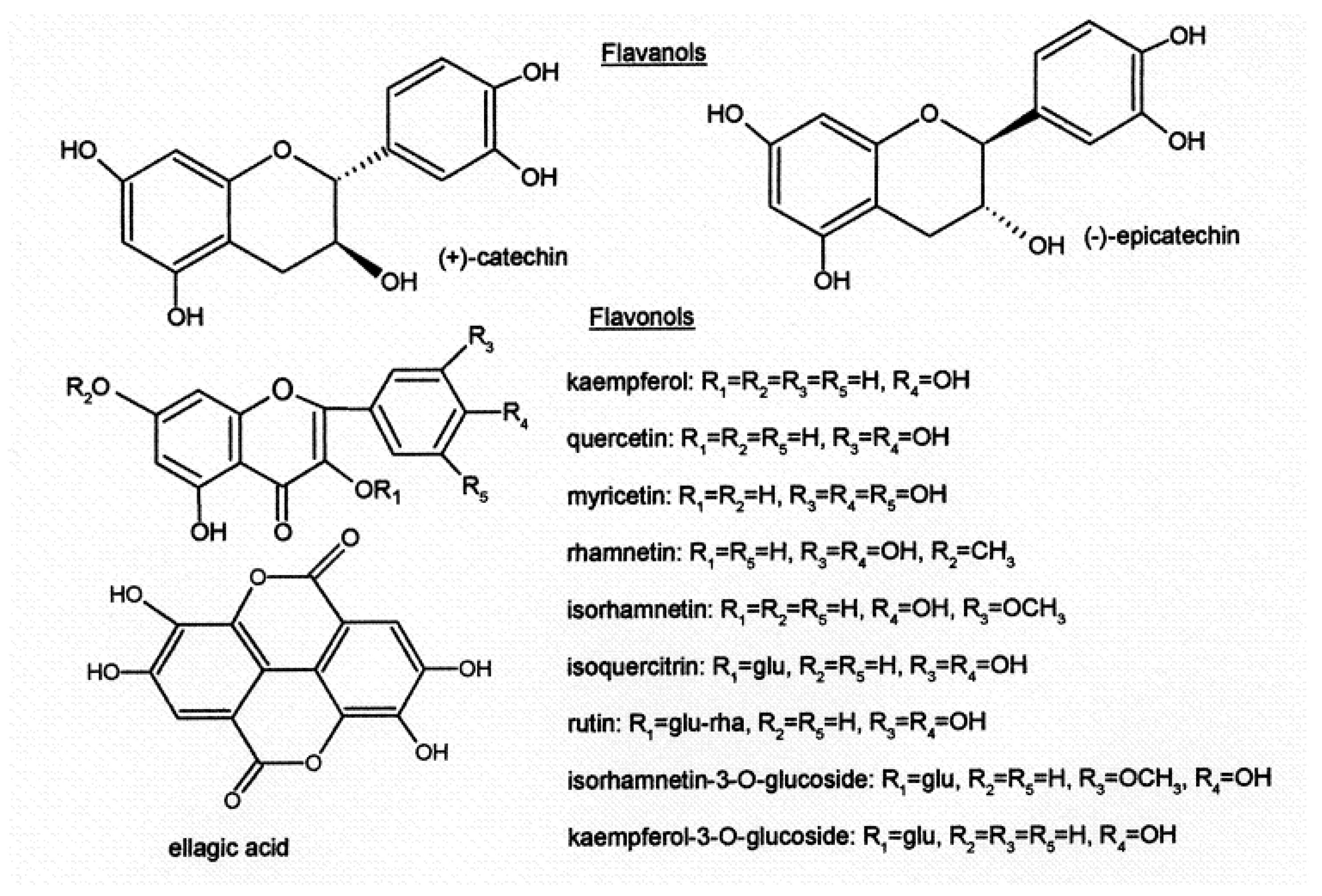
Another large group of flavonoids are flavonols (
Figure 4) (quercetin, myricetin, kaempferol, isorhamnetin and their glycosides), which contribute to bitterness, red wine color
[13], and antioxidant activity
[14]. The concentration of phenolic compounds in grapes is also dependent on the grape cultivar and influenced by viticultural and environmental factors, such as maturity stage, seasonal conditions, production area and fruit yield
[15][16][17][18].
Figure 4. Structure of flavanols, flavonols and ellagic acid.
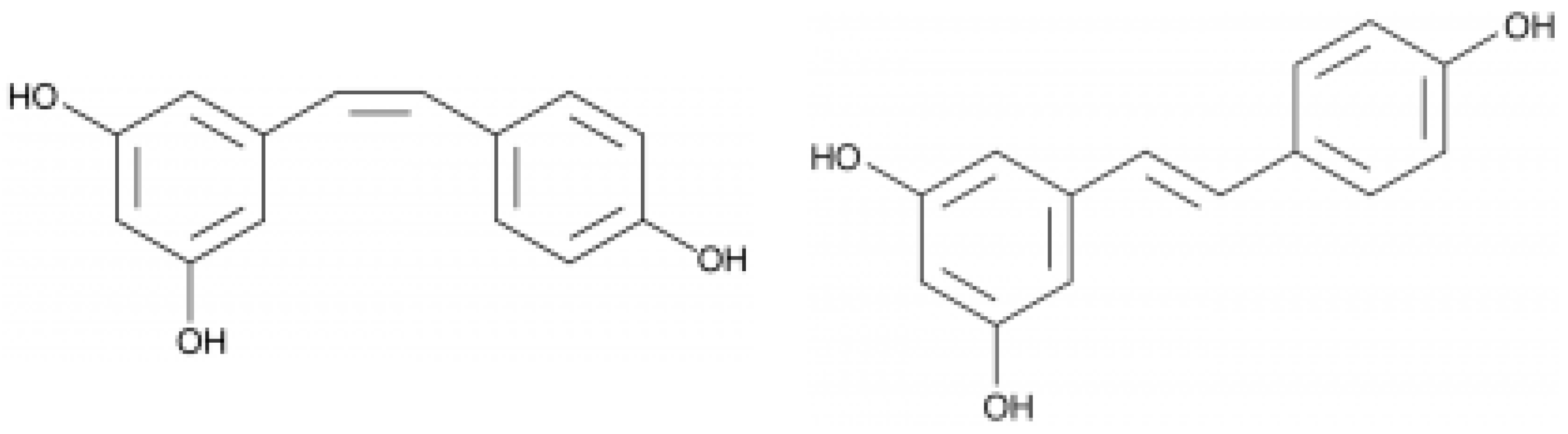
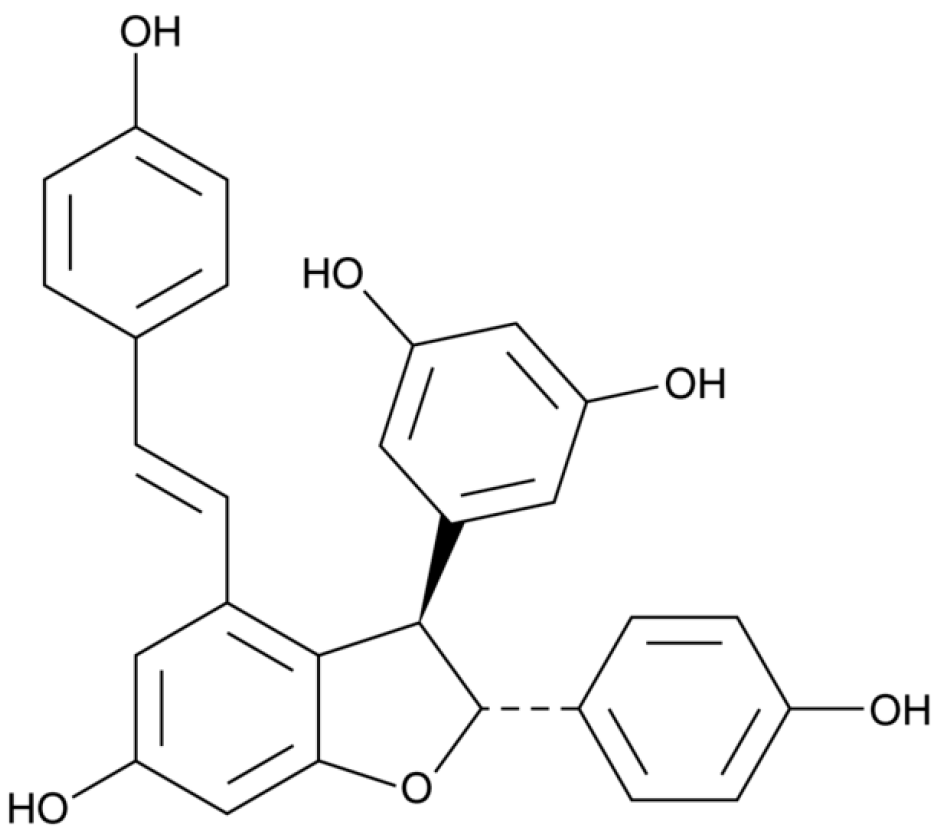
Resveratrol is synthesized in grape skin as a response to fungal infection (
Figure 5). The compound acts as a phytoalexin, preventing pathogen proliferation. During an attack of Botrytis cinerea (the main fungal infection damaging wine crops), plants form a resveratrol barrier
[19]. Additionally, in grape berries of some varieties, piceid, a stilbene glucoside of resveratrol is detected, which is related to the biosynthesis of resveratrol. Together with resveratrol, its oligomers (the dimer trans-ε-viniferin and trimer α-viniferin) have been detected in wine (
Figure 6). Resveratrol levels in red wines range between 0.1 and 14.3 mg/L
[20].
Figure 5. Structure of cis (left) and trans (right) resveratrol.
Figure 6. Structure of dimer trans-ε-viniferin (2) components of wine.
Table 1 and
Table 2 present the main grape and wine phenolic antioxidants (including phenolic acids) and their classification
[15][18][21][22][23].
Table 2. Phenolic compounds in different parts of grape and its products.
4. Health Effects of Wine Polyphenols
The phenolic components of wine have garnered significant research interest due to their antioxidant properties and potential beneficial effects on human health
[24]. Grape seed extract has been commonly utilized in recent years as a nutritional supplement
[25]. However, the analysis of phenolic compounds from vine and wine products (grape seeds and skins, musts, and wines) is complex due to their significant diversity. The dietary intake of polyphenols from red fruits, vegetables, and red wine can reach up to 200 mg/day, and their consumption via red wine has been proposed as part of the reason underlying the “French Paradox”
[26]. This suggests that a diet rich in saturated fats and moderate alcohol consumption could prevent the elevated levels of heart disease, cancer, and stroke found in other countries. Anthocyanins are effective antioxidants
[27] and possess other biological activities with health benefits independent of antioxidant capacity. This includes inhibition of cancer cell growth in vitro
[28], induction of insulin production in isolated pancreatic cells
[29], a reduction in starch digestion by inhibiting α-glucosidase activity
[30], suppression of inflammatory responses
[31], and protection against age-related decline in cognitive behavior and neuronal dysfunction in the central nervous system
[32]. The breeding of crops with increased anthocyanin content has been a significant target of research
[33]. However, to achieve biological effects in specific tissues or organs, anthocyanins must be bioavailable, meaning they are effectively absorbed from the gastrointestinal tract (GIT) into the circulation and delivered to the appropriate locations within the body. Studies on the oral administration of anthocyanins have confirmed the increased antioxidant status of serum
[34][35], but this is usually accompanied by a very low uptake of anthocyanins and corresponding low levels of urinary excretion as intact or conjugated forms. The apparent low bioavailability of anthocyanins casts doubts on the ability to exert their proposed beneficial effects in the human body. Therefore, anthocyanins are not generally recognized as a physiological functional food factor. However, cyanidin 3-glucoside (C3G), a typical anthocyanin, is reported to exert antioxidative and anti-inflammatory effects in vitro and in vivo
[36][37][38][39][40], clearly suggesting beneficial effects beyond its antioxidant capacity. Epidemiologic studies have linked flavonoid-rich foods with a reduced risk of cancer and cardiovascular disease. While the mechanisms underlying the suggested health benefits of flavonoid-rich foods remain to be fully elucidated, in vitro and in vivo studies have demonstrated that flavanols and procyanidins from wine have several beneficial biological activities, including the ability to reduce oxidative damage, promote endothelium-dependent relaxation, and decrease platelet aggregation.
4.1. Metabolic Syndrome
Metabolic syndrome is a combination of several clinical features including central obesity, high blood pressure, and elevated fasting glucose and triacylglycerol contents, along with low concentrations of HDL cholesterol, and insulin resistance. The clustering of these features is speculated to increase the risk of cardiovascular disease, which is associated with each component. Consistent with this theory, recent studies have reported that metabolic syndrome markedly increases cardiovascular morbidity and mortality. Metabolic syndrome components include the following: (1) central obesity measured as waist circumference (102 cm for men and 88 cm for women), (2) high serum triacylglycerol (150 mg/dL), (3) low serum HDL cholesterol (40 mg/dL for men and 50 mg/dL for women), (4) hypertension (systolic/diastolic pressure of 130/85 mmHg, and (5) high fasting glucose (110 mg/dL). Metabolic syndrome is defined as the presence of three or more of these components.
4.2. Alcohol Intake
Limited studies to date have focused on the effects of alcohol on the development of metabolic syndrome. While an association between alcohol drinking and prevalent metabolic syndrome has been documented, the findings are inconsistent. Some studies indicate that the relationship is inversely linear, J-shaped, or positively linear, whereas others show no association. In addition, the association appears to differ based on type of alcoholic beverage. Compared with no alcohol consumption
[41], light to moderate drinking of wine and beer appears favorable for reducing the prevalence odds ratio of metabolic syndrome, whereas liquor drinking tends to increase the ratio or have no association with metabolic syndrome. Earlier studies on the association between alcohol consumption and metabolic syndrome have had limited success in establishing causality owing to their cross-sectional design. To evaluate the effect of alcohol on the development of metabolic syndrome, the incidence of metabolic syndrome was prospectively examined in relation to alcohol consumption status, including average daily amount consumed, type of alcoholic beverage most consumed, and drinking frequency
[42]. Additionally, a prospective study on a Korean cohort aged 40–69 years showed that heavy drinking, particularly liquor, is associated with an increased risk of metabolic syndrome by affecting its components, including waist circumference, triacylglycerol content, blood pressure, and glucose. Although mounting evidence strongly supports beneficial cardiovascular effects of moderate red wine consumption (one to two drinks per day; 10–30 g alcohol) in most populations, clinical advice to abstainers to initiate daily alcohol consumption has not yet been substantiated in the literature and must be considered with caution on an individual basis
[43]. Further research is warranted to clarify the association between the level of alcohol consumption and metabolic syndrome risk, as well as the beverage-specific association in terms of beer or wine.
4.3. Coronary Heart Disease (CHD)
Coronary heart disease (CHD), also known as coronary artery disease, is the narrowing of the small blood vessels that supply blood and oxygen to the heart. CHD is usually caused by atherosclerosis, which occurs when plaque builds up on the walls of arteries, leading to narrowing. With the narrowing of coronary arteries, blood flow to the heart can slow down or stop, causing chest pain (stable angina), shortness of breath, heart attack, and other symptoms. Cardiovascular disease is the main cause of mortality in industrialized countries, but incidence rates show marked geographical differences. The low incidence of CHD in Mediterranean countries has been partly ascribed to dietary habits. Recent findings from studies on a large European cohort suggest that a high degree of adherence to the Mediterranean diet is associated with a reduction in mortality. In small-scale clinical studies, the Mediterranean diet or some of its components have been linked to reduced blood pressure along with improved lipid profiles
[44] and endothelial function. High blood pressure (HBP) is a serious condition that can trigger CHD and other health problems. Blood pressure refers to the force of blood pushing against the walls of arteries as the heart pumps out blood. A consistent increase in blood pressure over time can damage the body in many ways. Alcohol intake from any type of alcoholic beverage appears beneficial, but some studies suggest that red wine confers additional health benefits. The benefits of red wine are further supported by a meta-analysis of 13 studies involving 209,418 participants that showed a 32% risk reduction in atherosclerotic disease with red wine intake, which was greater than 22% risk reduction upon beer consumption. The dietary intake of flavanones, anthocyanidins and specific foods rich in flavanoids is potentially associated with a reduced risk of death due to cardiovascular heart disease.
Conversely, other investigations have failed to demonstrate the beneficial effects of red wine, leading to the conclusion that additional lifestyle factors, such as diet, exercise, socioeconomic status, or pattern of alcohol consumption potentially play a role in the lower rates of atherosclerosis in wine drinkers
[45][46]. However, increased alcohol consumption for the purpose of cardioprotection cannot be justified. There is no rational reason for non-drinkers to start consuming wine as a preventive measure considering that several other well-proven therapies exist for cardiovascular risk reduction, such as exercise, smoking cessation, blood pressure control, and the lowering of cholesterol
[47].
4.4. Dyslipidemia and Diabetes
High-density lipoprotein (HDL) is one of the five major groups of lipoproteins (chylomicrons, VLDL, IDL, LDL and HDL) that facilitate transport of lipids, such as cholesterol and triglycerides, within the water-based bloodstream. In healthy individuals, ~30% blood cholesterol is carried by HDL. HDL is proposed to remove cholesterol from atheroma within arteries for transport to the liver for excretion or re-utilization. Therefore, HDL-bound cholesterol is sometimes known as “good cholesterol” or HDL-C. A high level of HDL-C could protect against cardiovascular diseases and, conversely, low HDL cholesterol levels (<40 mg/dL or ~1 mmol/L) increase the risk of heart disease. Cholesterol contained in HDL particles is considered beneficial for cardiovascular health, in contrast to “bad” LDL cholesterol.
Recent attention has focused on food factors that may be beneficial in preventing body fat accumulation and reducing the risk of diabetes and heart disease. Although several drugs that target obesity-related metabolic diseases or prevent body fat accumulation have been marketed, little evidence showing that food factors are directly beneficial in improving the dysfunction of adipocytes responsible for adipocytokine secretion and lipid metabolism is available
[48]. Anthocyanins were recently shown to enhance adipocytokine (adiponectin and leptin) secretion, and the expression of PPARγ and adipocyte-specific genes in isolated rat adipocytes without stimulation via PPARγ ligand activity for the first time
[49]. However, other anthocyanin-responsive genes may exist that would contribute to clarification of the biological basis for utilization of anthocyanins as physiological functional food factors. Nutrigenomics is the application of high-throughput genomic tools in nutrition research. Significant advances in DNA microarray technology should promote our understanding of anthocyanin-mediated influence on gene expression and regulatory mechanisms of genes responsible for the prevention of obesity and amelioration of insulin sensitivity through modulation of adipocyte function. Data from DNA microarray analysis showed for the first time that anthocyanins enhance the lipolytic activity and gene expression of related enzymes in adipocytes
[50]. Dietary anthocyanin has recently been shown to significantly suppress the development of obesity. A few studies suggest that anthocyanins regulate obesity and insulin sensitivity associated with adiponectin and leptin secretion and PPARγ activation in adipocytes.
The normal non-diabetic blood glucose level ranges from 70 to 110 mg/dl depending on the type of blood tested. Glucose level > 140 mg/dL is usually indicative of diabetes (except in newborns and some pregnant women). Insulin, a hormone made by the pancreas, helps the body utilize glucose for energy. Insulin resistance is a condition in which the body produces insulin but cannot use it properly. In individuals with insulin resistance, the muscle, fat, and liver cells do not respond normally and require more insulin for glucose entry into cells. Eventually, the pancreas fails to keep up with the body’s surplus need for insulin. Excess glucose builds up in the bloodstream, setting the stage for diabetes. Patients with insulin resistance often have high levels of both glucose and insulin circulating in the blood.
Insulin resistance
[51] increases the risk of developing type 2 diabetes and heart disease. Atherosclerotic diseases are prevalent as secondary complications associated with type 2 diabetes, and a diet high in readily absorbable carbohydrates is associated with increased risk of type 2 diabetes
[52]. Accumulating epidemiologic data implicate postprandial hyperglycemia as a risk factor in the development of cardiovascular disease. Elevated postprandial glucose levels may have a direct toxic effect on the vascular endothelium mediated via oxidative stress, independent of other cardiovascular risk factors, such as hyperlipidemia
[53]. Postprandial hyperglycemia may also exert effects through its substantial contribution to total glycemic exposure. Ischemia-reperfusion causes oxidative damage that is enhanced with repetitive postprandial hyperglycemia
[54]. Among the cells damaged by diabetes are primary sensory neurons, also known as dorsal root ganglion neurons. Damage to these cells triggers diabetic peripheral neuropathy. Elevated glucose leads to apoptosis in neurons
[55] accompanied by increased oxidative stress. Procyanidins have insulin-like effects in insulin-sensitive cells that could explain their antihyperglycemic effect in vivo. These effects, in addition to their antioxidant activity, may contribute to beneficial effects against diabetes
[56]. Earlier epidemiologic studies indicate that alcohol consumption is associated with improved insulin sensitivity but experimental evidence to confirm this finding is limited. For instance, moderate wine consumption by overweight women in a previous study did not improve or impair insulin sensitivity or induce changes in any of the known indicators of insulin sensitivity, including body weight and composition, blood lipid, and blood pressure
[57].
This entry is adapted from the peer-reviewed paper 10.3390/foods13020295