Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Biomedical
Photoplethysmography (PPG) is used for heart-rate monitoring in a variety of contexts and applications due to its versatility and simplicity. These applications, namely studies involving PPG data acquisition during day-to-day activities, require reliable and continuous measurements, which are often performed at the index finger or wrist.
- photoplethysmography
- saturation
- wave morphology
1. Introduction
Cardiovascular diseases are the leading cause of death worldwide, with 17.9 million deaths each year [1,2], and there is a high prevalence of chronic cardiovascular disease that is the major cause of hospital admissions. Since chronic patients are ambulatory, there has been a growing interest in wearable devices capable of monitoring cardiovascular parameters on a daily basis [3]. Wearable devices are becoming a part of our daily routines, ranging from smartwatches to fitness trackers or even smart clothes [4,5,6]. Along with the commodities they offer, health monitoring technology has also branched into these devices, with the latest advances allowing for continuous health monitoring outside clinical settings [7]. At the same time, these devices are becoming increasingly accurate, meeting clinical standards [8,9]. Photoplethysmography is a must-have sensor in wearable devices for heart-rate monitoring, due to its simplicity, versatility, and non-invasiveness. Most often, these sensors are used at the fingertips due to their widely spread and shallow vascular bed [10], and, consequently, high signal amplitudes can be obtained [11]. While in an experimental setting these body parts are preferred, for lifestyle activity data collection they are impractical, and also much more susceptible to motion artifacts [8]. A caveat of PPG sensors is their tendency to saturate, which does not provide meaningful clinical information [12,13]. On top of this, when applying common biosignal filtering techniques, saturated portions lead to distorted filtered signal, a ringing effect, which in turn contributes to inaccurate peak detection and extracted features [12,14,15,16]. Consequently, alternative measurement sites need to be explored and validated to ensure that high-quality signals can be obtained. While many different anatomic regions are being used in research to obtain unsaturated data, a systemic analysis of the accuracy and quality of these new signals has not been made. Moreover, health parameters, such as heart rate (HR) and heart-rate variability (HRV), need to be accurately derived.
2. Photoplethysmography
Photoplethysmography is a non-invasive method of measuring blood volume changes in the human body. Incident light with a specific wavelength is used to extract optical properties from the microvascular bed of the skin and modulate the pulsatile blood flow [17].
PPG sensors consist of a photoemitter (PE) and a photodetector (PD). The light emitted from the source is absorbed, scattered, and reflected by the human body’s tissues. The PD’s position dictates whether the PPG is transmissive or reflective, as seen in Figure 1. In both of these types, the blood flow is modulated by the amount of light that is transiently absorbed by the skin. Transmissive PPGs have the PD attached across the body part, acquiring a signal based on the attenuation of the light crossing it. Reflective-type PPGs have the PD positioned side by side with the PE. In this case, the obtained signal comes from the scattering and reflection of the light. Transmissive PPGs are capable of more stable readings than reflective PPGs. However, reflectance mode PPG is more easily implemented, especially in wearable devices, since both the PE and the PD can be integrated in close proximity to each other [10,18].
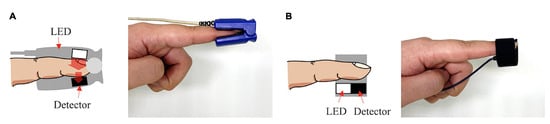
Figure 1. Transmissive PPG on the left (A), reflective PPG on the right (B).
The intensity of the captured light depends on various biological factors, such as tissue opacity of the interposed skin, connective tissue, bone, and also the amount of blood in the capillary vessels. Blood has a higher absorption coefficient than the other bodily components; therefore, variations in blood volume, such as arterial pulsation, account for the measurable cardiac cycle variations observed in the PPG signal [10].
Although different wavelengths have been used for PPG acquisitions, higher wavelengths can reach higher depths of penetration, with the wavelength of 660 nm being able to reach subcutaneous tissues. Red wavelengths, 640–660 nm, and infrared wavelengths, 880–940 nm, are commonly used for PPG readings [19]. Nonetheless, PPG sensors working in these ranges are significantly influenced by heat radiation from human skin. For instance, when comparing PPG signals using these wavelengths upon varying temperatures, the correlation between the signal and the biological feature being studied is weaker [20]. To overcome this, green wavelengths (around 525 nm) can be used, because they are not affected as much by both temperature effects and motion artifacts [19,20,21].
The PPG signal comprises two main components, the pulsatile or cardiac cycle (CCC) and superimposed or basal (BC) components. The pulsatile component originates from the blood volume variations created by the cardiac activity. As such, this signal portion depends on the cardiac phases, namely the systolic and diastolic phases. By isolating a single PPG pulse, four main fiducial points can be seen, as shown in Figure 2.
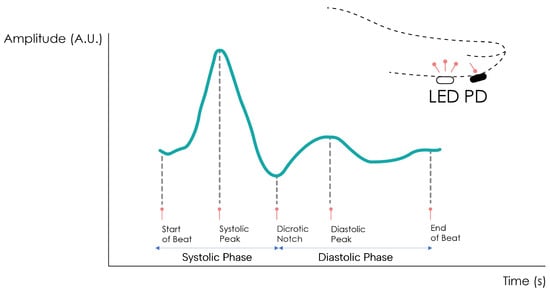
Figure 2. Normal PPG waveform of the CCC component.
The foot or onset corresponds to the beginning of the pulse (in the systolic phase), followed by the systolic peak, which is the maximum peak of the pulse signal, occurring after. The rising edge of the signal from onset to peak thus represents the rising blood volume within the measured body area. Next, a depression called the dicrotic notch occurs, as the aortic valve closes, followed by a lower peak, the diastolic peak. This second local maximum represents the reflected Windkessel wave [23,24]. The CCC component can thus be used to estimate cardiac-related features such as heart rate, heart rate variability, and blood pressure. Since these variations are synchronized with the cardiac cycle, they are also related to vasodilation, vasomotor, and vascular tones. The superimposed component, or nonpulsatile component, is mainly influenced by tissue composition and basic blood volume within the measurement site, and is often attenuated through signal conditioning [25]. This component is affected by internal parameters such as breathing, thermoregulation, and the activity of the sympathetic nervous system. Moreover, external parameters such as ambient light can also affect this signal component [10]. Because the amplitude of the PPG signal depends on the aforementioned internal and external factors, its measuring unit is considered arbitrary.
3. Measuring Sites
The most common measurement site for PPG signals is the finger. Being a peripheral part of the body with high blood perfusion allows for high signal amplitude [26]. The key disadvantage of this site is tied to the susceptibility of the sensor to motion artifacts. During daily activities, the fingers are constantly moving, creating a significant amount of motion noise which, in turn, compromises the measured signal [27]. Other measurement sites have been described in the literature, such as the earlobe, the wrist, the arm, or the ankle.
The earlobe has a much higher blood perfusion than the other measurement sites, even when compared to the finger, making it very attractive for obtaining high amplitude [28]. Earlobe sensors are also very easy to fabricate, but since the most common approach is with a spring-loaded ear clip, their use in long-term recordings becomes uncomfortable for the subject.
The wrist’s main advantages as a measurement site are its ease of use and discreetness. Many sensors are adapted into smartwatches, becoming a part of the subject’s usual garments, not requiring the volunteer to use any other equipment. The signal amplitude is lower than at the finger, but it is also less susceptible to motion artifacts [29,30].
The upper arm is also used despite the lower signal amplitude, due to its proximity to human arteries and its potential to be inserted into clothing, becoming just as comfortable as a wrist sensor [31].
The ankle, while less common, has a significant potential for implementing PPG data collection in discreet form factors. Being one of the farthest extremities of the body from the heart, it allows for the collection of peripheral blood perfusion data more accurately, helping detect conditions such as peripheral artery disease or peripheral vascular disease. It can also be easily integrated into undergarments such as socks to allow for constant monitoring without sacrificing comfort [22,32,33,34].
This entry is adapted from the peer-reviewed paper 10.3390/s24010214
This entry is offline, you can click here to edit this entry!
