Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Energy & Fuels
The intensive exploitation and usage of fossil fuels has led to serious environmental consequences, including soil, water, and air pollution and climate changes, and it has compromised the natural resources available for future generations. In this context, identifying new energy storage technologies can be considered a sustainable solution to these problems, with potential long-term effects.
- fossil fuels
- energy storage technologies
- material capabilities
1. Introduction
The transport industry is considered one of the main global consumers of natural resources, as well as the largest producer of greenhouse gas emissions, the effects of which contribute to accelerating the global warming phenomenon [1]. Currently, reducing automotive usage cannot happen suddenly in society; therefore, identifying alternatives for replacing non-renewable sources and decreasing generated pollutant emissions are promising future perspectives [2,3]. To stimulate the progress and transition toward the circular economy, the cost of developed technology is a significant variable, with a high impact on increasing the quality of life [4,5,6]. In addition, these alternatives should improve energy efficiency, increase energy conservation potential, encourage accessibility on a large scale, and decrease the environmental footprint of the entire process, from the design stage to recycling [7].
The implementation of these measures is necessary since climate changes are considered a significant threat to human evolution in the future. Therefore, any action that results in a reduction in anthropogenic impacts should be considered, optimized, and implemented [8,9,10].
Scientists are constantly looking to develop energy systems [11,12] that harness energy from renewable and sustainable sources, such as solar, wind, wave, or geothermal energy. Solar and wind energy are generally dependent on weather conditions, wave energy is harnessed from the oceans, and geothermal energy from rocks and fluids comes from the earth’s shell [13,14,15]. Therefore, it can be stated that these energy sources are location-dependent and difficult to store and transport.
For these reasons, a growing interest in hydrogen-based technologies has appeared in recent years. Hydrogen can be considered a renewable and abundant energy source and a “clean” fuel, which releases only water vapor into the environment during the electrochemical oxidation involved in the combustion process [16,17]. Moreover, hydrogen can be stored in portable and transportable systems, being an alternative energy source for motor vehicles. Hydrogen storage materials possess ecological and cost advantages, but the main bottlenecks are the high storage pressure, low volume concentration, and difficulties related to charging/discharging kinetics [18].
Another potential candidate for fossil fuel replacement is lithium-ion batteries, which stimulate the transition to electric vehicles [3,19]. This process involves numerous advantages but also has challenges that should be overcome. Even if lithium-ion battery technology has a fast evolution over time, the energy density has a lower value compared with conventional fossil fuels [20,21,22]. For commonly used vehicles, multiple solutions have been implemented with the main purpose of reducing total weight or increasing autonomy. But problems arise in the case of heavy vehicles, whose battery systems are too heavy to be considered practical [23].
2. Energy Storage System Evolution
The first reference to the fuel cell concept appeared in 1839, which represents the starting point for energy storage system development. British physicist Sir William Grove used the catalysis process to obtain electricity and water by mixing hydrogen and oxygen as fuels. This type of fuel cell operates with similar materials to today’s phosphoric acid fuel cell [27].
The ancestor of the rechargeable battery is the lead-acid battery. This early form of a rechargeable battery was developed in 1859 by the French physician Gaston Planté. For his initial model, Planté used two lead sheets separated with rubber strips and rolled into a spiral. The first application of this battery consisted of powering the lights in train carriages when stopped at a station [28].
Another step in the energy storage system evolution was the development of the flywheel, also known as the “mechanical battery”. This technology, used for energy storage in the form of rotational kinetic energy, began during the Industrial Revolution period. Flywheel energy storage was implemented in the military area starting in 1883 [29].
The nickel-cadmium battery, invented by Waldemar Jungner in 1899, consists of an anode made from a mixture of cadmium and iron, a cathode composed of nickel hydroxide (Ni(OH)2), and an alkaline electrolyte composed of aqueous KOH [30,31].
In 1907, Italy and Switzerland became the first countries to utilize pumped storage. This hydroelectric energy storage is based on water movement between two reservoirs that can generate power. This kind of system can both generate and store energy, so it can be stated that it is acting like an extremely big battery [32,33].
Sodium sulfur batteries were developed by Ford Motor Company in 1960 to power the new models of electric cars. This type of energy storage uses liquid sodium and liquid sulfur electrodes [34,35].
Superconducting magnetic energy storage, invented by M. Ferrier in 1970, uses superconducting coils to store magnetic energy [36].
The first borehole thermal energy storage (BTES) sites were developed in a xylem factory located in Sweden. This type of technology involves energy storage with a solid storage medium (rocks and sands) [37,38].
Compressed-air energy storage (CAES) technology was implemented for the first time in a power plant located in Huntorf, Germany, in 1978. Stored energy can be produced by coal and nuclear power plants. There are several systems, including the development of small-scale compressed air energy storage [39].
The supercapacitor, also known as the ultracapacitor, was developed by the Pinnacle Research Institute (PRI) in 1982. Its first applicability was in the military field [40].
One year later, in 1983, the researchers M. Skyllas-Kazacos and coworkers laid the foundation for the development of a vanadium redox flow battery at the University of New South Wales, Australia [41].
Also in 1983, R. Remick et al. were the first to perform scientific research on the bromine-polysulfide flow battery, which is a type of rechargeable electric battery [42].
In 1991, Sony and Asahi Kasei assembled the first lithium-ion battery [43].
In 2007, the paper battery, a different type of electric battery, with cellulose as a major constituent, was created by a group of students from Rensselaer Polytechnic Institute in Troy, New York [44].
3. Energy Storage Technology Grading
Energy storage technologies are classified into a variety of systems, which can be divided into five broad categories: mechanical, electrochemical (or batteries), thermal, electrical, and chemical storage technologies (Figure 1).
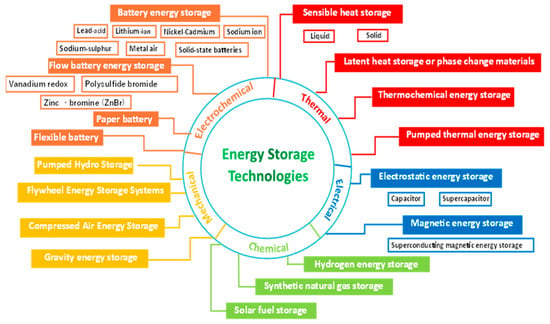
Figure 1. Energy storage technologies classification.
3.1. Mechanical Energy Storage System
Mechanical energy represents the energy that an object possesses while in motion (kinetic energy) or the energy that is stored in objects by their position (gravity energy). The exploitation of this type of energy using the power of heat, water, or air with turbines, compressors, or other systems, leads to the development of mechanical energy storage systems. In this case, the main systems are represented by pumped hydropower storage (PHS), flywheel energy storage systems (FESSs), compressed air energy storage (CAES), and gravity energy storage (GES) [45].
-
Pumped hydropower storage (PHS)
Pumped hydropower storage technology is based on two water reservoirs that are interconnected and located at different heights from each other. During surplus energy periods, the water is pumped from lower to higher reservoirs; therefore, mechanical energy is converted into potential energy. Extended demand leads to water release from the upper reservoir to the lower reservoir, making the hydraulic turbines operational and generating electricity [46].
-
Flywheel energy storage (FES)
Flywheel energy storage systems, which are considered mechanical batteries, have been used to store and transfer mechanical energy to and from the flywheel using an electric machine. The charging mode involves the use of electrical energy from the network in order to rotate the flywheel at very high speeds to generate kinetic energy, which will then be stored. In the case of the discharge mode, the rotor decelerates and the electric machine that acts similar to a generator converts the kinetic energy stored in the flywheel into electrical energy [48].
-
Compressed Air Energy Storage (CAES)
One of the many ways that energy can be stored for a long period of time is a compressed air energy storage system. Using CAES technology, the electricity surplus from the grid is used to compress the air with a rotary compressor and store it in an underground cavern. When the energy demand is high, the pressurized air is brought to the surface and passed through an air turbine that generates electricity [39].
-
Gravity energy storage (GES)
Gravity energy storage (GES) technology relies on pumped hydropower storage principles, which are based on storing electricity with potential energy. Therefore, the GES system operates by lifting heavy objects such as large masses of rocks, using excess energy available when the grid is disposed of extra energy. When energy is required, the objects are returned to their original position and potential energy is turned back into electricity with a generator [53].
3.2. Electrochemical Energy Storage (EcES) System
Electrochemical energy storage (EcES) systems are a traditional way to store energy for power generation. The chemical energy stored in this type of system is converted back into electrical energy when this is necessary. There are three categories of EcES systems that can be classified as batteries, electrochemical capacitors, and fuel cells.
-
Battery energy storage (BES)
Battery energy storage represents the most common type of EcES system. They are made up of two electrodes, an electrolyte, and a separator. The electrodes store the chemical energy, and the electrolyte allows the ions to flow between the electrodes. When the battery is discharged, the chemical energy is converted into electrical energy [56].
There are two types of batteries: primary and secondary. Primary batteries, the so-called single-use batteries, are characterized by the non-reversibility of electrochemical reactions [57]. On the other hand, a secondary battery is a device that stores chemical energy and then converts it back into electricity in a reversible way.
3.3. Thermal Energy Storage (TES)
Thermal energy storage (TES) systems represent a process of storing energy by changing the temperature, phase, or chemical bonds of a material. The materials are stored at high or low temperatures in a thermal-insulated tank, depending on the desired operating temperature range. The stored energy can then be recovered and used for a variety of residential and industrial applications, such as space heating or cooling, hot water production, or electricity generation [71].
There are many different types of TES systems, but they all work on the same basic principle: heat is transferred into a storage medium where it is stored until it is needed. The storage medium can be a solid, liquid, or gas, and it can be stored in a variety of ways, such as in a tank, underground reservoir, or phase change material [85].
-
Sensible heat storage (SHS) systems
Sensible heat storage (SHS) is a thermal energy storage technology that stores heat by raising the temperature of a material (either solid or liquid). The amount of energy that can be stored depends on the specific heat of the material (Equation (1)).
The specific characteristics of sensible storage materials include large densities, ρ (kg/m3), large specific heats, cp (J/kg–K), and large temperature differences between the hot and cold states, TH–TC (K) [86].
Water is the most common liquid used for SHS, but other materials such as molten salts and rocks are also used. A storage tank and a heat exchanger are the principal components of which an SHS system is made. The storage tank is filled with the storage material, and the heat exchanger is designed to transfer heat between the storage material and the working fluid. The working fluid serves as a fluid that is used to heat or cool the required application. This type of energy storage system is designed to store heat from a variety of sources, such as solar energy, industrial waste heat, and geothermal energy. The stored energy can then be used to heat and cool buildings, generate electricity, and power industrial processes. SHS systems are relatively simple and inexpensive to obtain, and in addition, these systems have an important characteristic in this field: high energy storage density. However, one major inconvenience of SHS systems is that they can only store heat at a single temperature [87].
-
Latent heat storage (LHS) systems
Latent heat storage (LHS) is a thermal energy storage technology that uses the latent heat of phase change materials (PCMs) to store and release thermal energy. PCMs are materials that can absorb or release a large amount of heat as they change phase from solid to liquid, or vice versa [86].
LHS systems work by transferring heat to or from the PCM, causing it to melt or solidify. During the melting process, the PCM absorbs heat from the surroundings, and during the solidification process, the PCM releases heat to the surroundings. The amount of heat that can be stored or released by an LHS system depends on the latent heat of fusion of the PCM and the mass of the PCM. The most common materials used in this type of energy storage are water, paraffin wax, salts, and metals. Water is the simplest and most inexpensive latent heat storage material, but it has a relatively low melting temperature. Paraffin waxes have higher melting temperatures and latent heat of fusion, but they are more expensive than water. Salts and metals have even higher melting temperatures and latent heat of fusion, but they are the most expensive latent heat storage materials. However, some liquid/gas substances, such as nitrogen and oxygen, can also be used for latent heat storage, especially for grid energy storage applications [88].
-
Thermochemical energy storage (TCES) systems
Thermochemical energy storage (TCES) is a type of energy storage that uses reversible chemical reactions to store and release heat. This contrasts with other energy storage technologies, such as batteries and pumped hydro storage, which store energy in the form of electrical or mechanical energy, respectively [89].
TCES works by storing energy in the form of chemical bonds. During the charging process, heat is used to drive a chemical reaction that produces two or more products. These products can then be stored for long periods of time without losing any energy. When energy is needed, the products are reacted back together to release heat [89].
Among the advantages of TCES over other energy storage technologies are a very high energy density (storing a lot of energy in a small volume) and efficiency (round-trip efficiencies of up to 80%). Additionally, TCES systems can be used to store energy for long periods of time, even years [90].
There is a wide range of potential uses for thermochemical energy storage systems that include the storage of solar heat provided by the sun for use in space heating, water heating, and power generation. Another application of TCES systems is represented by waste recovery heat from industrial processes to be used in electricity generation. TCES has an essential role in maintaining a clean environment, by storing the excess electricity that comes from renewable energy sources [89,90].
However, TCES systems are still in development, and there are some challenges that need to be addressed before they can be widely deployed. One challenge is that some TCES reactions can be slow. Another challenge is that some TCES materials can be corrosive. Despite these challenges, TCES is a promising technology for energy storage. It has the potential to help us store and use renewable energy more efficiently and to reduce our reliance on fossil fuels [91].
-
Pumped thermal energy storage (PTES) systems
Pumped thermal energy storage (PTES) is a technology that offers a perspective on large-scale energy storage. This energy storage system is based on a heat pump that uses grid electricity to alternate heat from low-temperature storage tanks to high-temperature storage tanks, creating stored energy that can then be used to generate power as needed. The materials that make up the tank are very important because they must have the ability to store heat at very high temperatures. One of the typical tank materials is molten salt [92].
PTES systems typically work using a Joule-Brayton cycle, which is the same cycle used in gas turbine power plants. The cycle consists mainly of four steps: compression, heating, expansion, and cooling [93].
PTES systems have several advantages over other energy storage technologies. They are highly efficient with round-trip efficiencies of up to 80%. They can also store large amounts of energy, making them ideal for long-duration energy storage applications. Additionally, PTES systems are relatively low-cost and have a long lifespan [94].
3.4. Electrical Energy Storage (EES) Systems
Electrical energy storage systems conserve energy in an electric field instead of changing it into another form of energy. There are two types of EES technologies available, each with its own benefits and inconveniences: electrostatic energy storage systems and magnetic energy storage systems.
-
Electrostatic Energy Storage Systems
The most common type of electrostatic EES is the capacitor. Capacitors store energy by separating oppositely charged plates with a dielectric material. When the capacitor is charged, a voltage is created between the plates, which stores energy [95]. Among the benefits of an electrostatic energy storage system are high energy density due to the large amount of energy stored in a relatively small volume, high efficiency because this type of technology can store and discharge energy with very little loss, very quick response times to charge and discharge, and a long lifespan. On the other hand, electrostatic energy storage systems also have some inconveniences like high cost and limited power density [96].
Capacitors and supercapacitors are both electrostatic energy storage systems. The first category is the most common type of electrostatic EES. They are used in a wide variety of applications, including electronics, power electronics, and energy storage. On the other hand, supercapacitors, the second electrostatic energy storage system, are a type of capacitor with a very high energy density. Although research on supercapacitors is still in progress, supercapacitors have the potential to play a major role in future energy storage systems [95].
-
Magnetic Energy Storage Systems
Magnetic energy storage systems (MES) are devices that store electricity in the form of a magnetic field with minimal loss of energy. The most popular type of MES is the superconducting magnetic energy storage (SMES) system. The energy stored using SMES systems is created by the magnetic field obtained in a superconducting coil. The superconducting coil current increases during the charging phase and decreases during the discharging phase. Superconducting materials possess the characteristic of having zero electrical resistance, which allows SMES systems to store large amounts of energy with minimal loss [97].
MES systems are a promising technology for energy storage. They offer several advantages, such as high energy density, high efficiency, and fast response times. However, they are still relatively expensive and require cryogenic cooling. As the technology continues to develop and become more affordable, MES is expected to play an even greater role in the future of energy [98].
3.5. Chemical energy Storage (CES)
-
Hydrogen energy storage
Hydrogen can be used in energy storage technology in three forms, gaseous, liquid, and solid. Hydrogen gas can be compressed and stored in high-pressure tanks. This is the most common method of storing hydrogen, but it requires a lot of energy to compress the gas, and the tanks are heavy and bulky. Also, this element can be liquefied by cooling it to very low temperatures. Liquid hydrogen is much more energy-dense than compressed gas, but it is also more expensive and difficult to store. On the other hand, solid-state storage involves hydrogen can be stored in solid materials, such as metal hydrides and carbon nanotubes. Solid-state storage is still under development, but it has the potential to be the most efficient and cost-effective way to store hydrogen [71,99].
Hydrogen is considered a promising solution for integrating large amounts of renewable energy into the grid, as it can be used to store excess energy generated during periods of high renewable output, such as when the sun is shining or when the wind is blowing. Hydrogen energy storage has a series of advantages over other energy storage technologies. Hydrogen is very energy-dense, meaning that it can store a lot of energy in a small volume. It is also non-toxic, but one of the most important challenges of this technology is represented by the difficulty of storing hydrogen safely and efficiently [100].
Despite these challenges, hydrogen energy storage is a promising technology for the future. It has the potential to help us integrate renewable energy sources into the grid and to provide long-duration energy storage. Overall, hydrogen energy storage is a versatile and promising technology with a wide range of potential applications. As the technology continues to develop and costs come down, it is expected to play an increasingly important role in the global energy transition [17,100,101].
-
Synthetic natural gas (SNG) storage
Synthetic natural gas (SNG) is a gas produced from a variety of sources, including renewable energy sources such as solar and wind power, biomass, and fossil fuels. SNG can be stored in the same way as natural gas, in underground caverns and high-pressure tanks. An important advantage of SNG technology is the potential to store a lot of energy in a small volume. Another benefit of SNG storage is that it can be used to store excess renewable energy obtained throughout the daytime and then discharge the energy when required (for example, when decreasing renewable energy production occurs). This can help to balance the grid and reduce reliance on fossil fuels [71,102].
SNG storage can also be used to provide long-duration energy storage. This is because SNG can be stored for long periods of time without losing its energy content. This makes it ideal for applications such as seasonal energy storage and backup power generation. SNG storage is a versatile and promising technology with a wide range of potential applications, including grid-scale energy storage, transportation, industrial applications, and residential and commercial applications [103].
-
Solar fuel storage
Solar fuel is considered a synthetic fuel that is produced using solar energy. A wide range of sources are used to produce this type of fuel, such as water, carbon dioxide, and biomass. Solar fuel storage is a method for storing solar energy in the form of chemical fuels. This can be performed using a variety of processes, such as photocatalysis, thermochemical cycles, and artificial photosynthesis. Solar fuels are very energy-dense, non-toxic, and non-flammable. There are various categories of solar fuels, but some of the most promising are hydrogen, methanol, and formic acid. Hydrogen can be produced from water using solar energy and can be used in numerous applications, including transportation, power generation, and industrial processes. Methanol can be produced from carbon dioxide and hydrogen using solar energy, and it contributes to applications such as gasoline blends, fuel cells, and direct methanol fuel cells. Formic acid is obtained from carbon dioxide and hydrogen using solar energy and can be used in fuel cells, direct formic acid fuel cells, and as a hydrogen storage material. For instance, hydrogen can be stored in compressed gas tanks, liquid hydrogen tanks, or metal hydrides. Methanol can be stored in liquid tanks or the form of methanol-based hydrates. Formic acid can be stored in liquid tanks or in the form of formic acid-based hydrates. The challenges associated with this type of energy storage technology include the inefficiency of solar fuel production processes and the safety and efficiency of storage. Nevertheless, this versatile and promising technology has great potential to integrate renewable energy sources into the grid and to provide long-duration energy storage [8,71].
This entry is adapted from the peer-reviewed paper 10.3390/en17010140
This entry is offline, you can click here to edit this entry!
