1. Glycerol Production and Applications
Biodiesel—fatty acid methyl ester (FAME)—is an alternative fuel produced from vegetable oils, animal fats, waste fats, and other fatty feedstocks with suitable characteristics and properties. Typical feedstocks for biodiesel production are rapeseed oil, soya oil, sunflower oil, palm oil, and oil obtained from microalgae [
21]. Cattle/sheep/poultry tallow, animal oil, used cooking oil, fish oil, jatropha oil, and coke have also been used [
22,
23].
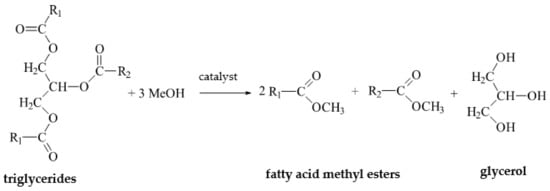
Biodiesel is synthesized by transesterifying lipids (triacylglycerols) with a simple alcohol, such as methanol or ethanol (alcoholysis) (
Figure 3). This reaction is usually catalyzed by NaOH, KOH, or sulfuric acid (VI) [
24,
25]. Chemical transesterification by alkaline catalysts is a fast-progressing reaction, which means that high volumes of triglycerides can be converted into their respective methyl or ethyl esters. The reaction will convert approx. 10% of the feedstock into glycerol [
26,
27]. However, it is difficult to separate the pure glycerol from the crude fraction due to the type and amount of impurities, such as methanol, fatty acid mono- and diacylglycerols, free fatty acids, and soaps produced during the biodiesel production process. Purified glycerol is a value-added product of great commercial and economic importance.
Figure 3. The triglyceride transesterification reaction.
Glycerol is also formed during bioethanol production via alcoholic fermentation [
28]. These biochemical pathways allow anaerobic microorganisms to regenerate the NAD (nicotinamide adenine dinucleotide) consumed during glycolysis. The pyruvate formed during the last step of glycolysis is then reduced to ethanol (ethyl alcohol) via a two-step process that also oxidizes the NADH (reduced form of NAD) formed during glycolysis to NAD while releasing carbon dioxide [
29]. In the first step, pyruvate is converted to ethanal (acetaldehyde) and carbon dioxide by pyruvate decarboxylase. The second step reduces ethanal to ethanol by alcohol dehydrogenase (ADH), with NADH oxidized to NAD [
30]. Alcoholic fermentation does not convert all of the glucose to bioethanol [
31]. A portion remains unconverted to bioethanol, while at the same time some of the bioethanol is subject to further reactions, which generate glycerol as a by-product [
32]. Yeast can also produce glycerol via osmotic regulation under stress [
33]. It is important to note that the glycerol output of bioethanol production can vary depending on a multitude of factors, including the type of feedstock, fermentation parameters, type of yeast, etc. [
34]. Bioethanol producers often strive to optimize their processes in order to minimize by-products, including glycerol [
35].
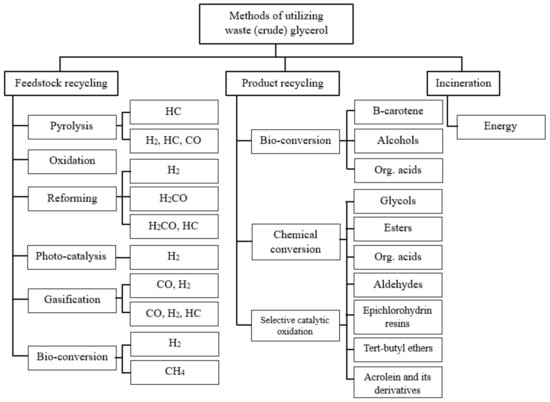
The efficient use of crude glycerol is crucial for the further commercialization of biodiesel production and can significantly reduce the price of biodiesel [
36]. One of the ways of neutralizing the waste glycerol fraction is to harness it for energy production, primarily by means of thermal conversion or bioconversion [
37,
38]. Major biofuel producers purify glycerin waste through filtration or fractional vacuum distillation [
39,
40], as well as by using chemical conversion or selective catalytic oxidation [
41,
42]. The literature also includes numerous reports on reusing the glycerin fraction in biological methods as a carbon source to fuel microbial growth. From a commercial standpoint, converting waste glycerol into gaseous biofuel via microbial bioconversion represents a major opportunity for the fuel industry. Well-selected microbial groups are capable of converting glycerol into value-added gas metabolites, and the process itself does not require that the glycerol fraction be purified [
43]. The cost-effectiveness of the process can be further boosted by replacing other carbon sources with cheaper waste feedstock. In this light, crude glycerol—seen as industrial waste and available in large quantities—would seem to be a good candidate for the production of biogases with various compositions and properties [
44,
45]. A basic division of the methods used to valorize and reuse waste glycerol from biodiesel production is presented in
Figure 4 [
37,
38,
43].
Figure 4. Primary uses of crude glycerol, own elaboration based on data from [
37,
38,
43].
2. Biomethane Production
A common method for neutralizing waste glycerol is by processing it via an anaerobic digestion (AD) process. AD utilizes biological conversion processes occurring under aerobic conditions, producing CH
4-rich biogas and digestate (usually utilized as organic fertilizer) [
46,
47,
48]. Each step of the digestion is mediated by specialized microorganisms that hydrolyze polymeric substances through enzyme action [
49,
50,
51]. They are further broken down into fatty acids, alcohols, H
2, and CO
2 [
52]. AD is a process that consists of the following steps: hydrolysis, acidogenesis, acetogenesis, and methanogenesis. Hydrolysis involves the enzymatic biological degradation of complex organic compounds. Enzymes produced by anaerobic, hydrolytic microorganisms are used in this process [
53]. During acidogenesis, the hydrolysates are further degraded into simpler compounds, mainly organic acids, alcohols and aldehydes, CO
2, and H
2 [
54]. Acetogenesis is the process by which organic acids are converted to acetic acid, CO
2, and H
2. Acetate is synthesized via one of two pathways. The first oxidizes the fatty acids produced in the acidogenesis phase, releasing H
2; in the second phase, it uses H
2 to reduce CO
2. Acetogenesis is the most important phase of the AD process [
55]. Methanogenesis is the last phase of AD. CH
4 is produced via two pathways. In the first pathway, it is produced from acetic acid, and in the second from H
2. In addition to CH
4, methanogenesis also produces CO
2, H
2S, ammonia, and water [
56]. The CH
4-producing organisms involved in this process are archaea [
57]. A step-by-step conversion diagram for the anaerobic digestion (AD) of glycerol (including the end products) is shown in
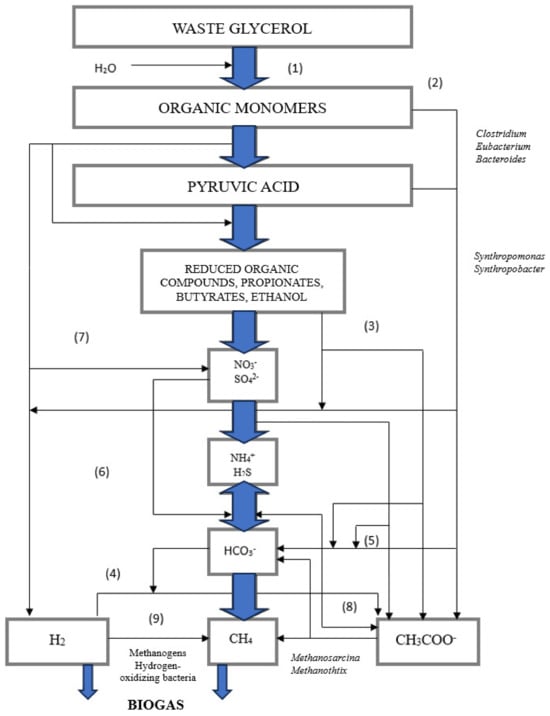
Figure 5.
Figure 5. Process flowchart for the AD of waste glycerol, own elaboration based on data from [
58,
59]. (1) organics are hydrolyzed, (2) monomers are digested, (3) propionic and butyric acids are oxidized by obligate H
2 producers (acetogenic bacteria), (4) hydrogen carbonates are oxidized by acetogenic bacteria, (5) propionic and butyric acids are oxidized by sulfate-reducing and nitrogen-reducing bacteria, (6) acetic acid is oxidized by sulfate-reducing and nitrogen-reducing bacteria, (7) H
2S is oxidized by nitrogen-reducing bacteria, (8) acetic acid is decarboxylated, and (9) CO
2 is reduced to CH
4.
Waste glycerol is usually included as a part of a wider mix of organic feedstocks that can be readily biodegraded in anaerobic digesters. The process by which bioreactors are fed with a mixture of different feedstocks is called co-digestion [
60]. Many previous studies and data from full-scale plants have shown amendment with waste glycerol to have a very positive effect on the AD process and its performance [
61]. This is owing to the high energy value of the substance, which increases the organic load rate (OLR) in the digesters while also directly and significantly boosting biogas yield and quality profile [
62]. Glycerol is readily biodegradable under anaerobic conditions, though it does typically require higher hydraulic retention times (HRT) in digesters [
63]. In many cases, its incorporation into the organic feedstock mix results in optimal OLR values and improves the carbon-to-nitrogen (C/N) ratio, which in turn reduces the levels of ammonia—a major inhibitor of AD in bioreactors [
64].
It has also been shown that waste glycerol-based processes produce digestate of better fertilizing quality [
65]. If the digestate from glycerol (co-)digestion is to be reused as an organic fertilizer, humus balancing in the soil becomes an important consideration [
66]. This is an important factor when growing cereals, rapeseed, root crops, and tuber crops—plantations that cause a negative organic matter balance in the soil. Such organic fertilizer can be successfully used in humus-poor, low-quality soils, including light, degraded, or marginal soils. The organic-rich digestate from glycerol digestion can be used as a first-line treatment for restoring the humus index, which has an enormous impact on soil productivity, improving the water and sorption (ion binding) capacity, structure, and biological activity [
67]. The literature often references the concept of “residual effect”, which refers to the crop yield increase upon the use of organic fertilizers that cannot be achieved with mineral fertilizers. The residual effect—such as the one from digestate derived from AD of glycerol—improves the physical, chemical, and biological properties of the soil [
67].
Because of the growing supply of waste glycerol and its promising properties as an AD feedstock, an increasing number of researchers and industrial-scale plant operators have been striving to optimize its use [
68]. Mesophilic digestion is the most popular AD process for this purpose, as evidenced, for example, by the studies on mesophilic co-digestion of sewage sludge and glycerol [
69,
70]. Research has shown that the best results are achieved when glycerol accounts for 25 to 60% of the total OLR fed into the digestate. Keeping the proportions within this range can boost biogas production by 82–280%. Increasing the share of glycerol in total OLR beyond 70% inhibited the process and hamstrung the result, i.e., biogas yields, CH
4 fractions, and digestate profile [
71,
72]. Baba et al. [
73] compared the energy balance of anaerobic digestion in a digester with an active volume of 30 m
3, fed with a mix of crude glycerol and excess sludge. Once a day, 3.5 m
3 of sludge and 5 to 75 L of crude glycerol were fed into the reactor. The best-performing variant provided a net energy output 106% higher than when digesting sewage sludge alone. The added benefit of the process was that the soil fertilized with the digested sludge showed improved structure and yielded 20% more grass for haylage.
Athanasoulia et al. [
74] used a continuous stirred-tank reactor (CSTR) to co-digest waste glycerol and sewage sludge under mesophilic conditions. The addition of up to 4% glycerol (
v/
v) improved biogas production by 3.8–4.7 times. Another study investigated the impact of glycerol addition on biogas yields at a pilot scale [
75] and found a low dose of glycerol (0.63%
v/
v) to be the optimal one. This variant had a CH
4 yield of 1.3 m
3/L crude glycerol [
75]. Silvestre et al. [
76] studied the effect of adding crude glycerol to AD-treated sewage sludge under thermophilic and mesophilic conditions. The addition of glycerol at the thermophilic temperature range had a negative impact on the stability and performance of the process, even at low doses. In contrast, the process performed steadily within the mesophilic temperature range, with a 148% increase in CH
4 output with 1% glycerol in the influent
v/
v (27% of influent COD). The addition of glycerol to sewage sludge can be used to effectively balance the C/N ratio. However, no improvements in biogas yield were observed when the glycerol content exceeded 1%
v/
v in the influent, likely due to the high C/N [
76].
These results suggest that crude glycerol can be used as a co-feedstock for the AD of sewage sludge, although various parameters should be taken into account—depending on the glycerol characteristics and operating conditions—to ensure digester stability and optimal performance [
76]. Another study [
77] concluded that feeding glycerol at up to 2%
v/
v did not enhance methane production. A detailed analysis of the process kinetics and biochemical dynamics of the microbial community found synergisms between glycerol and sewage sludge, with the result being improved biogas and CH
4 yields. Doses of up to 2%
v/
v glycerol provided a good balance between increasing the organic loading rate and minimizing the impact on hydraulic retention time. During continuous operation over 200 days, feeding glycerol at up to 2%
v/
v sharply increased the organic load by 70% and resulted in a 50% increase in CH
4 production, with nominal yields of 1248 ± 58 mL CH
4/L/d [
77].
Alves et al. [
78] investigated how the addition of 1 and 3% crude glycerol
v/
v from biodiesel production affected the AD primary sewage sludge. Process stability and performance were monitored using such parameters as pH, COD, VS, C/N, and volatile acid/alkalinity ratio. At 1% glycerol, the CH
4 yield was 223.8 mL CH
4/g VS, whereas at 3% glycerol, the CH
4 output was 368.8 mL CH
4/g VS. This translates to an increase in CH
4 yield of 61% and 167%, respectively, against the sludge-only control (138.2 mL CH
4/g VS). Peak daily biogas production (56.8 mL CH
4/g VS) was achieved with the highest glycerol fraction. However, an initial instability period (with methanogenesis inhibition) of 5.8 days was noted, likely attributable to the accumulation of intermediate volatile acids [
78]. Another study by Alves et al. [
79] aimed to evaluate the production of biogas (including CH
4) from the anaerobic co-digestion of primary sludge (PS) from sewage treatment, food waste (FW), and crude glycerol. To study the effect of glycerol on AD stability and performance, biochemical methane potential (BMP) tests were performed at different glycerol concentrations—1 and 3%
v/
v. A modified Gompertz model was used to describe biogas and CH
4 production. The study demonstrated that the small increases in the organic load from glycerol addition led to significant improvements in biogas and CH
4 production. Although methanogenesis was temporarily inhibited in the BMP tests with the highest glycerol concentration—suggesting a longer adaptation period for methanogenic Archaea—CH
4 production was later restored and even maximized under these conditions. The biogas and CH
4 production reached 432.4 mL/g VS removed and 343.3 mL/g VS at 1% glycerol, respectively, and 692.6 mL/g VS
removed and 525.7 mL CH
4/g VS at 3% glycerol, respectively. This translates to an increase in CH
4 production of 45.4% and 122.7% (for 1 and 3% glycerol, respectively) compared to the results for PS + FW [
79].
Waste glycerol has also been co-digested with wastewater. Siles et al. [
80] co-digested wastewater from biodiesel production with 1%
v/
v glycerol, obtaining 310 mL of CH
4/g COD
removed. Anaerobic co-digestion was shown to require less clean water and nutrients, resulting in commercial and environmental benefits [
80]. Fountoulakis and Manios [
81] tested the co-digestion of olive mill wastewater and slaughterhouse wastewater with 1%
v/
v glycerol. CH
4 production was 1210 ± 205 mL CH
4/d, which represents a 731 mL CH
4/d increase compared to the no-glycerol variant (479 ± 38 mL CH
4/d) [
81]. The authors of [
81] also co-digested the wastewater organic fraction of municipal solid waste (40% fruit, 25% potatoes, 25% vegetables, 8% bread, and 2% paper) with 1%
v/
v glycerol. CH
4 yield amounted to 2094 mL CH
4/d—meaning an increase of 694 mL CH
4/d compared to the no-glycerol variant (1400 mL CH
4/d).
3. Biohydrogen Production
3.1. Photofermentation
H
2 meets the criteria of a low-impact energy carrier [
110,
111]. Its calorific value ranges from 10.8 to 12.75 MJ/Nm
3, making it suitable for use in heating, power generation, and air/car transportation [
112,
113]. Currently, H
2 is used on a limited basis, mostly in the refining industry, fuel cells, and space technology [
114]. The main barrier to use is the lack of viable methods of production and storage—ones that would be technologically feasible and cost-effective [
115]. Conventional H
2 production mainly consists of thermochemical methods, including combustion, gasification, pyrolysis, thermochemical liquefaction, or water pyrolysis [
116]. However, such solutions are energy-intensive, pollution-generating, and mired in high investment costs [
117]. It is estimated that almost 95% of H
2 is produced by converting fossil fuels [
118]. Biomass-based technologies, as well as methods that harness the biological processes of microorganisms, are becoming more and more viable as a means of H
2 production [
119]. They consist mostly of organic-feedstock fermentation, carried out by specialized groups of bacteria, or biochemical processes in the cells of selected microalgae species [
120]. Crude glycerol can be converted to H
2 via two different fermentation processes: photofermentation and dark fermentation. The former requires a light source to proceed.
Photofermentation is performed by anaerobic bacteria, mainly green/purple sulfur and non-sulfur bacteria such as
Rhodobacter sphaeroides,
Rhodobacter capsulatus,
Rhodobacter sulidophilu,
Rhodopseudomonas palustris,
Rhodopseudomonas sphaeroides, and
Halobacterium halobium [
121]. These species are capable of breaking down organic acids into H
2 and CO
2 [
122]. Nitrogenase—the primary photofermentative enzyme—is a metalloprotein complex responsible for the biological fixation of molecular nitrogen and commonly found in both archaea and bacteria. The nitrogen fixation process can produce H
2, thus maintaining redox homeostasis [
123,
124]. Nitrogenase activity can be inhibited by excessive C/N, oxygen, or ammonia, among other factors [
125]. Chemical energy in the form of a proton gradient is produced from sunlight. This energy can be used to power various cellular processes, including a reduction in nitrogenase via reverse electron flow. On the other hand, reduced nitrogenase uses the hydrolysis of ATP, produced by photophosphorylation, to reduce metabolically produced protons to H
2. The normal physiological function of nitrogenase is to reduce dinitrogen to ammonia, with the reduced release of one H
2 per N
2. However, nitrogenase turnover can still occur even in the absence of other reducible substrates, reducing protons to H
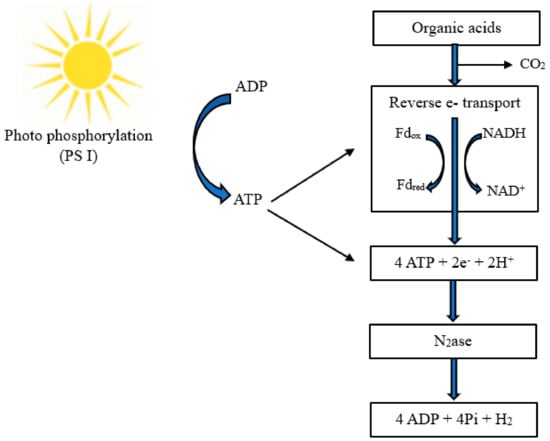
2. The process follows the flowchart presented in
Figure 6 [
122].
Figure 6. Flowchart of photofermentative H
2 production by microbes, own elaboration based on data from [
122].
The literature data indicates that
Rhodopseudomonas palustris is the most popular microorganism for the photofermentation of waste glycerol, owing to its particular enzymatic activity [
126]. Pott et al. [
127] found that an
R. palustris community fed with waste glycerol grew at a rate of 0.074 h
−1. In this particular process, glycerol was converted at a conversion efficiency of approximately 90% at a reaction rate of 34 mL H
2/g
dw/h. Inhibition of microbial growth and H
2 production was noted during the experiment, which was attributed to the contaminants in the crude glycerol. Saponified fatty acids were determined to be the main inhibitors of the process [
127].
Sabourin-Provost and Hallenbeck [
128] conducted a comparative study with both pure and crude glycerol as feedstock for photofermentative H
2 production by
Rhodopseudomonas palustris. The yields were approx. 6 moles of H
2/mole glycerol across all variants. The impurities present in crude glycerol were not found to negatively affect the process of microbial growth in this case [
128]. In another study, Ghosh et al. [
129] endeavored to optimize a photofermentative H
2 production process by
Rhodopseudomonas palustris. Response Surface Methodology (RSM) was applied to select the process conditions and parameters important for glycerol-to-H
2 conversion. This optimization protocol was used to determine the interactive effects among the process parameters, such as the concentration of crude glycerol and glutamate, light intensity, nitrogen source, and N levels, on H
2 production efficiency.
Under optimal conditions (light intensity of 175 W/m
2, 30 mM glycerol, and 4.5 mM glutamate), 6.69 moles H
2/mole of crude glycerol were obtained, which translates to 96% of the theoretical yield. Determination of nitrogenase activity and expression levels showed that process variables produced relatively little variation in nitrogenase protein levels, whereas nitrogenase activity varied considerably, with peak nitrogenase activity (228 nmol C
2H
4/mL/min) achieved at the optimal central point [
129]. Zhang et al. [
130] used modeling to simulate the entire growth phase of
R. palustris in order to maximize H
2 production. Two piecewise models were designed, both fitted via batch experiments, by solving the underlying optimal control problems through stable and accurate discretization techniques. The optimal initial glutamate-to-glycerol ratio was 0.25, regardless of the initial biomass concentration. The glycerol conversion efficiency was found to correlate with the initial biomass concentration, with a computational peak of 64.4%. By optimizing a 30 day industrial batch process, an H
2 production rate of 37.7 mL g biomass/h was achieved, while the glycerol conversion efficiency was maintained at 58% [
130].
3.2. Dark Fermentation
Bacteria-mediated dark fermentation is one of the most effective means of bioconverting biomass (including glycerol) to H
2 [
140]. The primary sub-types of such fermentation are butyrate/butanol fermentation, conducted by the genus
Clostridium sp., and mixed-acid fermentation, typical of the family
Enterobacteriaceae (
Enterobacter aerogenes,
Escherichia coli,
Vibrio cholerae,
Klebsiella pneumoniae,
Shigella dysenteriae),
Klebsiella sp.,
Bacillus sp.,
Clostridium pasteurianum,
Clostridium butyricum,
Thermotoga neapolitana, or mixed microbial communities [
141]. In commonly used technological processes, microbial cultures are fed with glycerol medium, containing waste glycerol, nutrients (nitrogen, potassium, and other compounds), and sometimes yeast. The process is conducted in an anaerobic environment at a temperature of approx. 30–40 °C [
142].
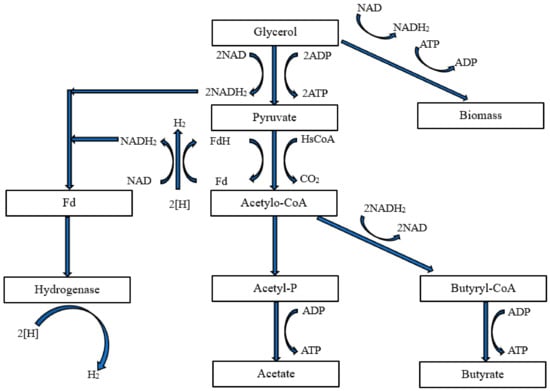
H
2 release by bacteria results from pyruvate molecules being broken down into acetyl-CoA. This reaction is catalyzed by pyruvate-ferredoxin oxidoreductase. Ferredoxin plays a key role in electron distribution within the cell as an electron carrier. Reduced ferredoxin can transfer electrons to iron-containing hydrogenase, allowing protons to be used as a final electron acceptor, thus facilitating H
2 production. An example flowchart of H
2 production by
Clostridium sp. bacteria is presented in
Figure 7 [
143].
Figure 7. Flowchart of glycerol-to-H
2 production by
Clostridium spp., own elaboration based on data from [
143].
Research to date has shown that the efficiency of glycerol-to-H
2 conversion via dark fermentation is not affected by impurities in the starting substrate, regardless of the degree of contamination [
141]. Zahedi et al. [
144] found that adding 1%
v/
v crude glycerol to the organic fraction of municipal waste almost doubled H
2 production in a process mediated by
Eubacteria and
Archaea. A trial using municipal waste as the sole feedstock resulted in a yield of 1.25 ± 0.29 LH
2/L. By comparison, H
2 production after amendment with glycerol (1%
v/
v) amounted to 2.32 ± 0.21 LH
2/L [
144]. Trchounian K. and Trchounian A. [
145] conducted a dynamic and kinetic analysis of glycerol-to-H
2 conversion by
E. coli. Metabolic engineering of
E. coli proved to directly improve dark fermentation efficiency. It was demonstrated that it was possible to redirect metabolic pathways, induce the DHAP production pathway, and block lactic, acetic acid, and ethanol production [
145]. Hu and Wood [
146] obtained an improved H
2-producing strain that yielded 0.68 mmol H
2L/hL in a glycerol medium. This represented a 20-fold increase in H
2 production compared to the precursor [
146]. What is more, Sanchez-Torres et al. [
147] and Tran et al. [
148] identified uncharacterized genes (54% of the
E. coli genome has been experimentally demonstrated; the remainder is uncharacterized or has been modeled through computational methods) that can be inactivated to boost H
2 production from glycerol. Some of the identified strains were able to produce 1.6 times more H
2 [
147,
148].
There have also been studies comparing
E. coli with other bacteria in terms of the optimal fermentation conditions, process parameters, and H
2 yields [
149]. Lo et al. [
150] tested crude glycerol from the biodiesel industry for H
2 production using seven isolated H
2-producing bacterial strains (
Clostridium pasteurianum,
Clostridium butyricum, and
Klebsiella sp.). Among the strains tested,
C. pasteurianum CH
4 had the highest H
2 production performance under the optimal conditions at a temperature of 35 °C, an initial pH = 7.0, an agitation rate of 200 rpm, and a glycerol concentration of 10 g/L. When using pure glycerol as the carbon source for continuous fermentative hydrogen production, the average H
2 production rate was 103.1 ± 8.1 mL/h/L, with yields of 0.50 ± 0.02 moles H
2/mole glycerol. By comparison, when crude glycerol was used as the carbon source, the H
2 production rate and H
2 yields rose to 166.0 ± 8.7 mL/h/L and 0.77 ± 0.05 moles H
2/mole glycerol, respectively [
150].
Kumar et al. [
151] used
Bacillus thuringiensis EGU45H-2 for fermentative hydrogen production from crude glycerol, which yielded H
2 at 0.646 mol H
2/mole glycerol consumed in a batch culture. Under continuous culture, after 2 days’ hydraulic retention time,
B. thuringiensis immobilized on lignocellulosic materials (banana leaves—BL, 10%
v/
v) produced 0.386 mol H
2/mole glycerol consumed. The maximum yield was 0.393 mol H
2/mole glycerol consumed [
151]. Ito et al. [
152] evaluated the production of H
2 and ethanol from glycerol-containing biodiesel waste using
Enterobacter aerogenes HU-101. H
2 and ethanol yields were inversely correlated with glycerol levels. The highest production of H
2 and ethanol—1.12 and 0.96 moles H
2/mole glycerol, respectively—was obtained at 1.7 g/L glycerol [
152].
Experimental reports confirm that co-digestion of mixed carbon sources—for example, formate and glycerol—can significantly increase H
2 production. Trchounian et al. [
153] found that H
2 production by
E. coli BW 25113 wild type, supplemented with glycerol, reached 0.75 ± 0.03 mmol H
2/L at the end of the exponential growth phase (7 h growth). The action of formate (10 mM) and glycerol (10 g/L) at pH 6.5 led to a yield of 0.83 ± 0.05 mmol H
2/L at the early exponential phase (2 h growth). The same stimulatory effect of formate and glycerol on H
2 production was observed at pH 7.5 [
153].
There have also been studies on how the type and concentration of heavy metals affect H
2 production and
E. coli growth rate [
154]. It was found that
E. coli possessed four [Ni-Fe]-Hyd enzymes, the activity of which requires heavy metals. Furthermore, Mo is required to activate formate hydrogen lyase (FHL). It was found that low concentrations of Ni, Fe, and Mo ions, as well as combinations thereof (Ni
2+ + Fe
3+ (50 mM), Ni
2+ + Fe
3+Mo
6+ (20 mM), and Fe3
+ + Mo
6+ (20 mM)), could increase bacterial biomass and H
2 production, mostly by way of glycerol fermentation under acidic conditions (pH 5.5 and pH 6.5). In contrast, Cu
+ and Cu
2+ (100 mM) were found to have no effect [
154].
Pachapur et al. [
155] verified the effect of added surface-active substances on H
2 production from glycerol in a mixed
Enterobacter aerogenes and
Clostridium butyricum culture. The process yielded up to 32.1 ± 0.03 mmol H
2/L medium under the optimized conditions of 17.5 g/L crude glycerol and 15 mg/L non-ionic surfactant (Tween 80). The increase in H
2 production was around 1.25-fold higher with Tween 80 than without, with a production of 25.56 ± 0.91 mmol/L [
155]. Faber and Ferreira-Leitão [
156] endeavored to optimize the conditions of the dark fermentation of waste glycerol. A statistical analysis showed that the optimal conditions were: pH = 5.5, glycerol concentration of 0.5 g/L, and volatile suspended solids of 8.7 g/L. H
2 production in the optimized process was 2.44 mol H
2/mole glycerol [
156].
H
2 is often combined with microbial fuel cells. Sharma et al. [
157] investigated the possibilities of converting glycerol into H
2 and electricity. Hydrogen bioreactors (HPB) and microbial fuel cells (MFC) were used for this purpose. A comparative analysis of pure and waste glycerol was carried out. A comparable H
2 production efficiency was achieved with 0.17–0.18 mol H
2/mol glycerol, regardless of the source of the substrate. The highest power density of 4579 mW/m
3 was achieved with 2 g/L of pure glycerol. The observed power densities of waste glycerol ranged from 1614 to 2324 mW/m
3 [
157]. Chookaew et al. [
158] conducted analog studies with a two-stage process combining dark fermentation with an MFC or a microbial electrolysis cell (MEC). The efficiency of H
2 production was 332 mL/L. 20% of the organic matter was biodegraded in the process. The two-chamber MFC produced a power density of 92 mW/m
2 and removed 50% of the COD. The columbic efficiency (CE) was 14%. When the cell was fed with 50% diluted fermentation product, similar power output (90 mW/m
2) and COD removal (49%) values were achieved, but the CE was almost doubled (27%). When similar substrates were used to produce H
2 in two-chamber MECs, the diluted influent provided better performance, with the yield reaching a maximum of 106 mL H
2/g COD and 24% CE. Thus, dark fermentation coupled with MFC/MEC proved to be a viable option for converting waste glycerol into bioenergy [
158].
Fermentative H
2 production from glycerol is influenced by many factors and system parameters, the most important being: glycerol type, origin and composition, presence of impurities, hydraulic retention time, type of digester, pH, temperature, and microbial strain [
159]. Even trace amounts of oxygen in the system can inhibit hydrogenase activity when obligate anaerobes are used. Therefore, it is usually a safer choice to use facultative anaerobes, such as
Clostridium sp. and
Enterobacter sp., which better tolerate oxygen in the bioreactor [
160]. The optimal pH range for efficient hydrogen-producing fermentation of glycerol is 5.0 to 6.0. Any increase in pH above that range can induce methanogenic bacteria to grow, consuming H
2 to produce CH
4 [
161]. On the other hand, lower pH values cause microbes to switch their metabolism towards other biochemical processes. This leads to a change in the composition of the gaseous products and impaired H
2 production. Additionally, a pH below 4.0 can inhibit microbial growth [
162].
3.3. Mixed Fermentation
A combination of dark and photofermentation has been used to enhance H
2 production [
169]. Dark fermentation produces alcohols and organic acids as by-products, which serve as a carbon source for the bacteria involved in photofermentative H
2 production [
170]. Integrated biological processes significantly improve the ratio of energy stored in H
2 to the energy needed to maintain the culture. The literature data show that ratios of up to 3.0 are achievable [
171,
172]. Chookaew et al. [
158,
173] studied H
2 production from crude glycerol and experimented with a two-step process involving dark fermentation followed by photofermentation [
174]. The strains used for the two-steps were
Klebsiella sp. TR17 and
Rhodopseudomonas palustris TN1, respectively. The optimal results of photofermentation were obtained with diluted wastewater, added glutamate, and no added yeast. Under these conditions, total H
2 production across the two-step process was 6.42 mmol/g COD—10.4% of the theoretical yield [
174].
Rodrigues et al. [
175] evaluated H
2 production in an integrated biosystem through dark fermentation followed by photofermentation, using crude glycerol from biodiesel production from waste cooking oils (WCO). A mixed
Clostridiales culture was able to take up crude glycerol during dark fermentation at 37 °C and pH 5.5, generating a high H
2 output of 22.38 mmol H
2/L with yields of 1.75 mol H
2/mole glycerol (which corresponds to 24.06 mmol H
2/g COD). The dark fermentation effluent (DFE) in concentrations of 1.0, 2.0, and 3.0 g COD/L was used to grow a phototrophic bacteria culture of the order
Rhizobiales and
Clostridiales at 37 °C, pH 7.0, and 18.50 W/m
2. The optimum conditions were achieved with 1.0 g COD/L DFE, generating 3.94 mmol H
2/g COD
consumed, and removing 76.10% COD. The culture was able to absorb ethanol (76.86%), acetic acid (95.73%), butyric acid (94.76%), and particularly methanol (99.18%) [
175].
4. Biohythane Production
Coupling H
2 production with CH
4 production—a solution known as “biohythane”—can optimize the reuse of leftover organic matter from fermentative hydrogen production and thus help recover gas energy [
187]. The leftover spent media from dark fermentative H
2 production contains high levels of short-chain fatty acids such as acetate, butyrate, propionate, etc., which could be a suitable feedstock for methanogens [
188]. Before the spent media are subjected to biomethanation, their pH should be adjusted to a range of 7 to 7.8. Additionally, the H
2 dissolved in the media also promotes the growth of hydrogenotrophic methanogens. The recovery of gaseous energy in the form of H
2 alone might not be enough to make the process commercially viable, since only 20–30% of total energy can be recovered through H
2 production. In this light, integrated biohythane production seems to be a good choice since it provides an attractive method of converting organic residues rich in carbohydrates, lipids, and proteins for clean energy generation. On the other hand, these systems present challenges of their own, including the shading effect of pigments produced by photofermentative organisms and problems with scale-up [
188].
Two-step AD has been acknowledged to be a promising method of biohythane production, as it enables reduced organic loading and increased total energy conversion efficiency by generating two gases with high fuel-burning capacity [
189]. By separating the hydrolysis/acidogenesis phase from the methanogenesis phase, the stability of the whole process can be improved—controlling the acidification phase at the H
2 generation stage (first stage) can prevent the inhibition of the methanogen population during the methane generation phase (second stage) [
190]. Two-step biohythane production has been carried out using feedstocks such as maize silage [
191], algal biomass [
192], food waste [
193], dairy waste [
194], organic fraction of municipal sewage wastewater [
195], cassava stillage [
196], tequila vinasses [
197], and skim latex serum [
198]. There are few reports on the use of glycerol for biohythane production, even though glycerol is a very promising substrate due to its high content of carbon and macronutrients. Jiang et al. [
199] conducted a two-step AD process to degrade glycerol. The system produced H
2 and CH
4 yields of 0.026 L H
2/g COD and 0.29 L CH
4/g COD
removed, respectively. In addition, 93% COD removal was noted [
199].
Co-digestion with glycerol can boost biohythane production at the optimal C/N range of 19 to 41 and the right H
2/CH
4 ratio of 0.06 to 0.3 [
200]. Jehlee et al. [
200] used glycerol as a co-substrate with
Chlorella sp. TISTR 8411 biomass for biohythane production. Mono-digestion of
Chlorella sp. TISTR 8411 biomass produced H
2 and CH
4 yields of 36.4 mL H
2/g VS and 166.18 mL CH
4/g VS, respectively. Co-digestion of
Chlorella biomass brought a marked improvement in biohythane production. The optimal dose of 2% TS glycerol waste resulted in 39.8 mL H
2/g VS and 577.33 mL CH
4/g VS being produced [
200]. Dounavis et al. [
201] developed a continuous process for the production of biohythane from crude glycerol in a two-step reactor system. In the first step, H
2 production was tested using mixed acidogenic communities in an up-flow column bioreactor. Cylindrical ceramic beads with a porosity of 600 m
2/L were used as an attachment matrix for bacterial cells. The experimental parameters were: HRT 24 h; pH 6, 6.5, and 7; and a glycerol concentration of 20 g/L. The effluent from the hydrogenic reactor was fed to a methanogenic continuous stirred reactor (CSTR), which was used to examine the effect of organic loading on CH
4 yield. The gaseous phase of the reactors was mixed to produce biohythane. At 20 g/L glycerol and 6, 6.5, and 7 feedstock pH, the H
2 output was 0.051, 0.070, and 0.094 L/g COD, respectively. CH
4 was produced at yields of 0.257 L/g COD (commercial glycerol), 0.283 L/g COD (crude glycerol), 0.198, 0.242, and 0.273 L/g COD (effluents from the hydrogenogenic stage (1st stage), diluted with water to 5, 7.5, and 10 g COD/L, respectively) [
201].
This entry is adapted from the peer-reviewed paper 10.3390/en17020338