Primary ovarian failure (POF) is caused by follicle exhaustion and is associated with menstrual irregularities and elevated gonadotropin levels, which lead to infertility before the age of 40 years. The etiology of POI is mostly unknown, but a heterogeneous genetic and familial background can be identified in a subset of cases. Abnormalities in the fragile X mental retardation 1 gene (FMR1) are among the most prevalent monogenic causes of POI. These abnormalities are caused by the expansion of an unstable CGG repeat in the 5′ untranslated region of FMR1. Expansions over 200 repeats cause fragile X syndrome (FXS), whereas expansions between 55 and 200 CGG repeats, which are defined as a fragile X premutation, have been associated with premature ovarian failure type 1 (POF1) in heterozygous females. Preimplantation genetic testing for monogenic diseases (PGT-M) can be proposed when the female carries a premutation or a full mutation.
1. Introduction
Preimplantation genetic testing for monogenic diseases (PGT-M) has marked a turning point in the approach to reproductive counseling and clinical practice for genetic disorders. However, both technical and clinical difficulties remain, many of which are specific to the underlying genetic condition. Among the most challenging in this regard are
FMR1-related disorders, in which PGT-M procedures and counseling are conditioned both by the complexity of the genetic locus and the limited availability of biological substrates due to accelerated exhaustion of the ovarian reserve in specific subsets of carriers.
2. Premature Ovarian Failure
Premature ovarian failure (POF), also known as premature ovarian insufficiency, is determined by depletion of follicles in the ovaries, which leads to infertility before the age of 40 years [
1]. This disorder arises with amenorrhea or oligomenorrhea for at least 4 months, increased gonadotropin levels, and decreased estrogen levels [
2]. Although the majority of POF cases are considered idiopathic, at least a proportion of them can be attributed to three distinct, recognizable causes: chromosomal abnormalities, autoimmune alterations, and monogenic disorders [
3].
Chromosomal abnormalities are among the most common genetic causes: numerous karyotype anomalies have been found in POF patients, including 45,X-Turner syndrome, 45,X/46,XX or 45,X/47,XXX mosaics, X-deletions, X-autosome translocations, X-isochromosomes, and other rearrangements [
3]. POF can also be observed as part of the clinical presentation of several autoimmune disorders, including Addison’s disease, Sjögren’s syndrome or dry-eye syndrome, autoimmune polyglandular syndrome, rheumatoid arthritis, systemic lupus erythematosus, and myasthenia gravis [
4].
Regarding hereditary forms, over 50 genes have been found to be involved in the etiology of POF, the most representative genes being
FOXL2,
CLPP,
FSHR, and
FMR1 [
3,
5]. Mutations in
FOXL2 have been associated with POF, in the form of blepharophimosis, ptosis, and epicanthus inversus syndrome. Ovarian failure has been also described, together with sensorineural hearing loss, in Perrault syndrome, for which four responsible genes have been identified:
C10ORF2,
CLPP,
HARS2, and
LARS2. Mutations in the
FSHR gene have been known to cause gonadotropin-resistant ovary syndrome, typically characterized by primary amenorrhea (or oligomenorrhea), menopausal follicle-stimulating hormone (FSH) levels (>40 mIU/mL) before the age of 40, and impaired follicle growth [
6].
3. FMR1
The human X chromosome fragile site (Xq27.3) coincides with a hypermethylated CpG island in the 5′ UTR of
FMR1 [
9,
10,
11,
12,
13].
FMR1 encodes the FMRP protein, which is expressed in many organs; the highest expression is in the brain, where it is expressed in differentiated neurons, and testes. FMRP plays an important role in the translational regulation of other cellular transcripts. The CpG island contains a polymorphic trinucleotide (CGG) repeat sequence, normally ranging between 5 and 44 repeats.
Recent studies established that AGG interruptions within the CGG repeat sequence might act as a “protective factor” against the risk of intergenerational expansion. In particular, women whose premutated allele contains at least one AGG interruption within the CGG repeat sequence have a lower probability of expanding their CGG repeat length into a full mutation and are therefore less likely deliver a child with FXS in the next generation [
20,
21,
22].
Regarding FMR1 premutation carriers specifically, it should be noted that, other than the risk of expansion in subsequent generations, they also display a diverse spectrum of health disorders, entailing wide and impactful implications for the proband and family members of all ages.
4. POF1
POF1 is a chronic disorder characterized by oligo/amenorrhea and hypergonadotropism before the age of 40 years [
33] that affects approximately 13–20% of female carriers with the premutation [
34,
35].
The first group to hypothesize a correlation between
FMR1 and POF were Cronister et al. [
36]. Welt et al. introduced the use of the term fragile X primary ovarian insufficiency (FXPOI) for patients whose ovarian dysfunction could be associated with premutation of the
FMR1 gene [
37]. In the following years, the development of more precise diagnostic tools allowed a more in-depth analysis and differentiation of
FMR1 alleles. Bodega et al., supported the correlation between
FMR1 expansion and POF, suggesting that the onset of ovarian dysfunction could be affected both by the pattern of interruption of the CGG repeat and X-inactivation [
38].
However, the association between premutation repeat size and the risk of ovarian failure has been found to be not linear [
42]. Allen et al. conducted a study on a very large cohort in which they detailed the reduction in AAM years in relation to CGG repeat size. They showed that women with midrange premutation repeats were at the highest risk of POF1 and premature AAM. Women with a repeat size of 70–120 experienced a 5-year reduction in AMM, while women with 85–89 repeats had the highest risk of ovarian failure, with an average AMM ~10 years earlier than <45 repeats group. Women with full mutation alleles, on the other hand, seem to have the same risk of POF as the general population (1%) [
43].
The role of intermediate alleles, which have an estimated prevalence in the range of 0.8–3% in the general population [
44], has been more extensively debated. Previous studies had shown that even women carrying
FMR1 intermediate alleles (45–54 repeats) were at risk of developing POF [
8,
38,
39,
45,
46].
Overall, the lower limit of
FMR1 repeat sizes that modify ovarian function has not yet been established [
40]. Patients carrying expansions falling within the intermediate range can exhibit milder forms of premature ovarian senescence, defined as diminished ovarian reserve (DOR) and, for the most part, characterized only by the premature elevation of baseline FSH concentrations. However, it has been observed that not all women with ovarian dysfunction and DOR are bound to develop POF [
24,
34,
39,
51,
52,
53,
54,
55].
5. FMR1 Premutation and Ovarian Damage
To date, the mechanisms of ovarian damage caused by FMR1 premutation are not yet completely understood. In women carrying the full mutation with an expansion of over 200 CGG repeats, the FMR1 allele is usually hypermethylated and therefore transcriptionally silenced. For this reason, the lower level of FMRP observed in premutation carriers is not considered a plausible cause of POF, which is typically not present in individuals with fully expanded alleles.
Several authors [
61,
62,
63,
64,
65,
66,
67] suggest that accumulation of pathological
FMR1 mRNA might be responsible for POF1 through a toxic gain-of-function effect in ovarian cells [
67,
68]. More specifically, they hypothesized that expanded CGG repeats might lead to the formation of dynamic intra-nuclear long rCGG RNA aggregates capable of binding and sequestering crucial RNA binding proteins.
Another notable factor to consider is the existence of AGG triplets interrupting the CGG repeat sequence, which could represent a marker for a better fertility outcome for women carrying a premutation. CGG repeats in the
FMR1 mRNA are known to form stable secondary structures, primarily in the shape of hairpins [
70]. The effect of AGG interruptions could be mediated through inhibiting the formation of secondary structures at the DNA level.
6. Reproductive Counseling and Fertility Preservation
Genetic counseling concerning the risk of disease transmission and available reproductive choices should be offered to all women carrying the
FMR1 premutation or full mutation before they reach reproductive age [
71]. In particular, it should be carefully discussed that the
FMR1 premutation is associated with the risk of reduced ovarian reserve in ~16% of carrier women [
24,
34,
35,
39,
40,
51,
52,
53,
54,
55] and that carriers with the premutation, or even an intermediate number of CGG repeats, have a higher risk of developing POF1 [
67]. Even if complete depletion of the ovarian reserve does not occur, carriers might still suffer from infertility or sub-infertility [
34,
57,
72,
73,
74].
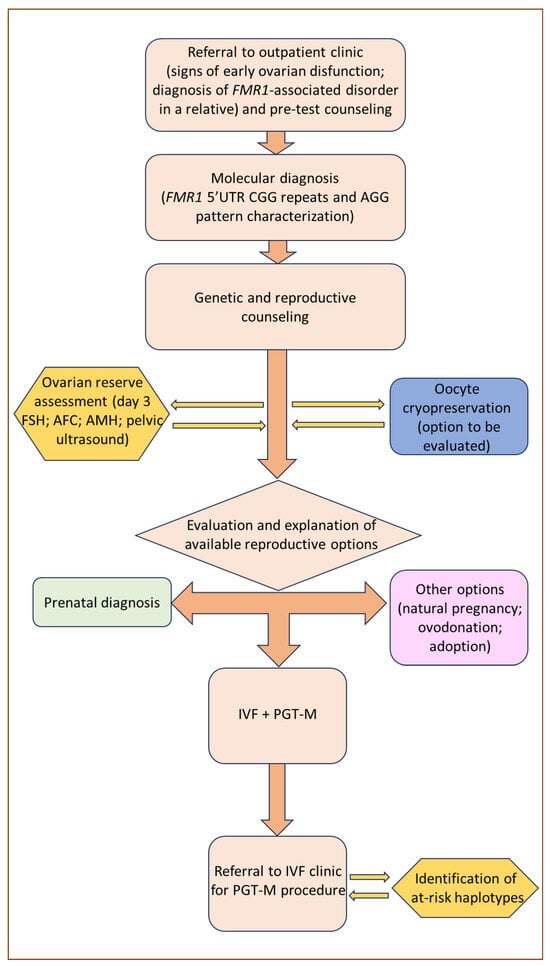
Among the reproductive options for couples with a family history of FXS, the most established historically is prenatal diagnosis, the outcome of which enables couples to choose, after the appropriate genetic counseling, between the continuation and voluntary termination of pregnancy. However, prenatal genetic counseling in FXS families is complicated by the difficulty of accurately predicting the phenotype of unborn children, which can vary significantly due to X-inactivation ratios in women, mosaicism in men, and intergenerational allele expansion. Furthermore, prenatal diagnosis followed by the voluntary termination of pregnancy is often experienced as an emotional burden [
78]. Therefore, it is not surprising that alternative approaches, such as PGT-M, have progressively become a preferable option, although with certain limitations. A graphical exemplification of the counseling process for women suspected to be carriers of the
FMR1 premutation and who might express their inclination towards PGT-M is presented in
Figure 2.
Figure 2. Clinical management flow diagram for women referred on suspicion of being
FMR1 premutation carriers.
7. PGT-M Strategies and Limitations in FMR1-Related Disorders
For many years now, preimplantation genetic testing for monogenic diseases (PGT-M) following an IVF procedure has been considered a valid alternative to prenatal diagnosis, by providing the opportunity of achieving pregnancy with a healthy child without the psychological stress caused by voluntary termination [
79,
80]. Standard IVF treatments followed by PGT-M begin with controlled ovarian stimulation protocols by gonadotropins administration, ultrasound-guided transvaginal route follicular aspiration, and subsequent in vitro intracytoplasmic sperm injection (ICSI).
In the context of FMR1-related disorders, the aim of PGT is the selection of female embryos with both normal alleles, and male embryos hemizygous for the normal maternal allele. The PGT strategy can be theoretically proposed to any couple at risk of transmitting FXS.
Regarding the analytical procedure, its main limiting factor is the number of CGG repeats, both in the healthy and in the pathological allele, which is particularly relevant when trying to distinguish healthy and carrier female embryos. Whenever a PCR-based sequencing technique is employed in FMR1 preimplantation testing, the number of CGG repeats on the healthy FMR1 allele of the carrier mother needs to be different from the number of repeats on the FMR1 allele of the father.
Standard PCR amplification methods are therefore unsuitable for approximately one-third of PGT couples, whose normal alleles happen to be identical in size. In these otherwise non-informative couples, DNA markers linked to the
FMR1 gene can be used whenever sufficient data on family haplotypes are available [
83,
84,
85,
86,
87].
8. POF1 in the PGT-M Context
The main biological limitation to PGT-M in
FMR1 disorders concerns female premutation carriers, due to their higher risk of POF in comparison to individuals with either normal or fully expanded alleles [
24].
FMR1 premutation carriers show lower response to ovarian stimulation, even when administered higher gonadotropin doses. They also exhibit lower peak estradiol levels, fewer retrieved oocytes, and lower rates of successful blastulation in comparisons with controls [
35,
91].
Although some authors considered PGT-M for
FMR1-related disorders as an inefficient and therefore unviable option [
85], nonetheless several PGT centers routinely make their services available to full mutation or premutation carriers. Literature regarding the effectiveness of ovarian stimulation and oocyte retrieval is abundant, but also contradictory. Two different groups, led by Platteau and Avraham, effectively showed that ovarian stimulation of premutation carriers required a higher dose of FSH, and observed a higher risk of oocyte retrieval failure due to insufficient ovarian response [
80,
93].
Since ovarian reserve is a prime determinant of IVF success, Pastore and colleagues [
48] also studied the effect of
FMR1 CGG expansion length on IVF outcomes following controlled ovarian hyperstimulation (COH). In their meta-analysis, the authors demonstrated that
FMR1 premutation carriers (CGG 50–200) had lower success rates, as measured by oocyte yield, following IVF treatments than women with normal CGG repeat length or a full mutation, although with some conflicting results. The association between the number of CGG repeats and patients’ response to COH was also inconsistent [
48].
9. Conclusions
FMR1 premutation is among the most important monogenic causes of POF. For this reason, the analysis of
FMR1 CGG expansions in women with signs of ovarian dysfunction of unknown cause may prove to be a decisive and appropriate diagnostic approach. The analysis should include the assessment of
FMR1 gene structure, including both CGG repeats and AGG interruption pattern. Moreover, such a diagnosis in young women allows appropriate genetic counseling for planning their reproductive prospects considering possible ovarian dysfunction. The estimation of ovarian reserve or any COH variable is also important. The repercussions of premutation, intermediate expansion, and full
FMR1 mutation on the response to ovarian stimulation and the subsequent efficiency of PGT-M outcomes remain under debate [
79]. Regarding reproductive options, PGT-M for POF1 is not an obvious choice for at-risk couples and it should be preceded by a thorough discussion on its risks, limitations, and benefits. As long as oocytes and embryos are successfully obtained, premutation carriers might have a good chance of pregnancy [
97] and their pregnancy rates are not statistically different with respect to full mutation patients [
79]. However, poor ovarian response, as well as technical limitations of the diagnostic procedure, may preclude specific subsets of at-risk couples from accessing the technique.
This entry is adapted from the peer-reviewed paper 10.3390/genes15010006
 Encyclopedia
Encyclopedia
