Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Chemistry, Analytical
Formaldehyde, a ubiquitous indoor air pollutant, plays a significant role in various biological processes, posing both environmental and health challenges.
- formaldehyde
- electrochemical sensor
- enzyme
- electrocatalyst
1. Introduction
Formaldehyde, represented by the chemical formula CH₂O, stands as a fundamental organic compound and the simplest aliphatic aldehyde. The carbon–oxygen double bond imparts significant polarity to formaldehyde, making it highly reactive in chemical reactions. Additionally, formaldehyde exhibits the typical characteristics of an aldehyde group, facilitating a range of specific reactions. Formaldehyde finds extensive applications in various industrial sectors, including resin production, antimicrobial agents and disinfectants, textile processing, pharmaceutical manufacturing, biomedical research, furniture, and building materials [1,2,3,4,5]. However, the extensive use of formaldehyde has raised alarms due to its role as a significant indoor air pollutant, classified as the third-largest by the World Health Organization in 2004 [6].
Additionally, formaldehyde is a crucial bioactive molecule intricately involved in various biological processes. Within plant organisms, formaldehyde can be generated through pathways involving methyl transfer and demethylation [7]. As part of chemical communication, plants release formaldehyde as a response to environmental stress [8]. Furthermore, formaldehyde serves as a secondary metabolite produced during the synthesis of specific compounds, potentially endowed with defensive or protective functions. Confronted with external pressures such as pathogen infections, climate variations, or damage, plants may produce formaldehyde as part of their stress response mechanism. In summary, the generation of formaldehyde within plant organisms involves multifaceted processes encompassing physiological activities, metabolic pathways, and interactions with the external environment, potentially playing a crucial role in plant growth, development, and adaptability [5]. In animals and the human body, endogenous formaldehyde holds significance with various physiological functions, primarily generated through metabolic reactions. These reactions are especially orchestrated by enzymes such as semicarbazide-sensitive amine oxidase (SSAO) and cytochrome P450 in coordinated demethylation reactions. SSAO, a copper-dependent amine oxidase, catalyzes the deamination of compounds like methylamine, resulting in the production of formaldehyde [9]. This enzyme is widespread in organs such as the brain, heart, and liver. Cytochrome P450, on the other hand, participates in enzymatic reactions aimed at breaking down foreign substances, thereby aiding in the production of formaldehyde to eliminate extraneous materials. Importantly, cellular organelles like the endoplasmic reticulum play a pivotal role in the formation of formaldehyde, including processes like succinate-enhanced formaldehyde accumulation. Additionally, various components in the cell nucleus or cytoplasm can transform into formaldehyde, contributing to biological methylation and other essential processes [10]. Moreover, formaldehyde plays a crucial role in cellular signal transduction by covalently binding with proteins and nucleic acids, thereby regulating their activity and stability [11]. This modification process is implicated in various biological processes, including cell growth, differentiation, and apoptosis, underscoring the key role of formaldehyde in maintaining cellular signal balance and functional stability [12].
However, exposure to an environment with excessive formaldehyde and elevated levels of formaldehyde in the human body can have severe consequences. Such exposure can lead to discomfort in the eyes, respiratory problems, skin irritation, and adverse effects on the nervous and immune systems [13]. The concentration of formaldehyde in human blood varies between 10 μM and 100 μM, and higher accumulation of formaldehyde is associated with organ aging [14]. Elevated formaldehyde concentrations can intensify apoptotic activity or reduce mitotic activity in cells [15]. Research has also identified that excessive formaldehyde exposure can trigger various diseases, including chronic inflammation, fetal development issues, cardiovascular diseases, leukemia, and nasopharyngeal cancer [16,17,18,19]. Therefore, the detection of formaldehyde is crucial and has sparked extensive research. Accurately monitoring formaldehyde levels is essential for protecting human health and preventing potential risks in the environment. Moreover, research in this field is continually expanding, exploring more advanced and sensitive detection technologies to gain a more comprehensive understanding of the distribution and impact of formaldehyde in different environments. Through an exploration of the physicochemical properties of formaldehyde, a variety of detection methods have been developed, including gas chromatography [20], high-performance liquid chromatography (HPLC) [21,22], infrared spectroscopy [23], Raman spectroscopy [24,25], colorimetric methods [26,27], fluorescence spectroscopy [28,29,30,31,32], mass spectrometry [33,34], electrochemical methods [35,36,37,38,39,40,41], and quartz crystal microbalance [42,43], among others. Each of these methods possesses its own unique characteristics. For instance, chromatography methods offer high accuracy but involve complex operational procedures and relatively slow detection speeds, limiting real-time monitoring capabilities. Spectroscopic methods, on the other hand, provide rapid response times, particularly fluorescence analysis, which allows for in situ real-time monitoring. However, spectroscopic methods often require the use of derivatization or fluorescent probe molecules, necessitating the specific design and synthesis of responsive molecules.
2. Sensing Mechanisms of Electrochemical Formaldehyde Sensors
Electrochemical formaldehyde sensors based on solution reactions can be primarily categorized into three types based on their electrode reaction mechanisms. The first category encompasses enzymatic formaldehyde sensors, which rely on biological enzymes for formaldehyde detection. The second category comprises electrochemical sensors that utilize electrocatalysts (i.e., inorganic metals or metal oxides) to catalyze the oxidation of formaldehyde. Finally, the third category encompasses electrochemical sensors derived from specific chemical molecules. These distinct sensor types offer various advantages and applications, contributing to the versatility and effectiveness of formaldehyde detection methods.
The operational principle of enzymatic formaldehyde electrochemical sensors relies on the highly specific catalytic activity of enzymes. These sensors primarily consist of a working electrode modified with a specific enzyme, typically formaldehyde dehydrogenase (FDH), which is specialized in catalyzing the oxidation of formaldehyde. The electrode’s operational process is illustrated in Figure 1.
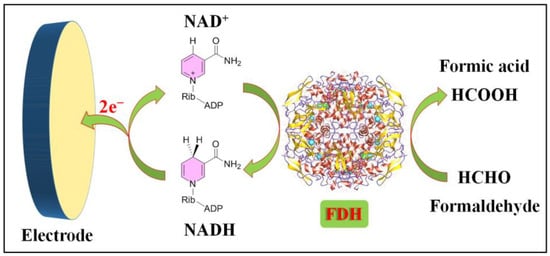
Figure 1. Formaldehyde electrochemical sensors rely on bioenzymes.
Another crucial approach to developing formaldehyde sensors involves the electrocatalytic oxidation of formaldehyde using different electrocatalysts (Figure 2). With the advancement of nanomaterial synthesis and characterization techniques, an increasing number of such electrochemical formaldehyde sensors have emerged over the past decade. These sensors primarily employ various electrocatalysts, including elemental metals, metal alloys, metal oxides, hydroxides, heterogeneous materials, and non-metallic electrocatalysts. Depending on the type and properties of the electrocatalysts, which encompass variations in the redox potentials of metal species, the electronic conductivity of materials, and their adsorption capacity for formaldehyde and oxidation intermediates, the process of catalyzing formaldehyde oxidation, as well as the resulting catalytic products, can differ.
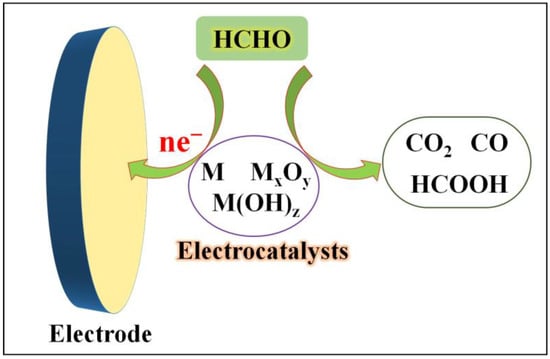
Figure 2. Formaldehyde electrochemical sensors rely on electrocatalysts.
Formaldehyde exhibits versatile chemical properties due to its active carbonyl group and hydrogen atoms. Firstly, the carbonyl group’s high electrophilicity allows it to react with organic compounds such as thiols and amines, resulting in various chemical reactions, including aldehyde–amine condensation, 2-aza-Cope rearrangement, Pictet–Spengler reaction, and Hantzsch reaction. Secondly, formaldehyde possesses reducing properties and can reduce specific metal ions to their anionic forms while oxidizing itself to formic acid. Additionally, formaldehyde can react with bases to form methanol salts. These distinct chemical reactivity characteristics of formaldehyde have paved the way for the development of electrochemical sensors. There have been reports of electrochemical sensors based on formaldehyde derivatization reactions [57,58,59]. Typically, these sensors utilize specific derivatization reagents that selectively react with formaldehyde, leading to the formation of probe molecules with new electrochemical properties upon interaction with formaldehyde (Figure 3). This approach enables highly selective formaldehyde detection and has been extensively applied in fluorescence-based assays. However, its application in electrochemical detection is relatively limited, indicating the potential for further exploration in this area.
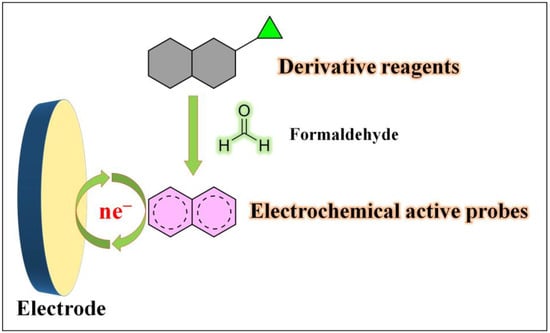
Figure 3. Formaldehyde electrochemical sensors rely on derivative reagents.
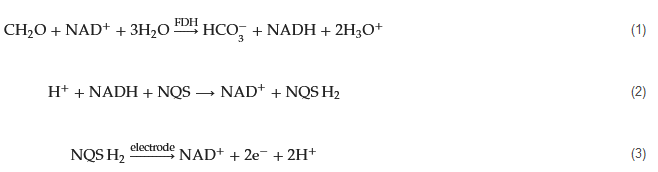
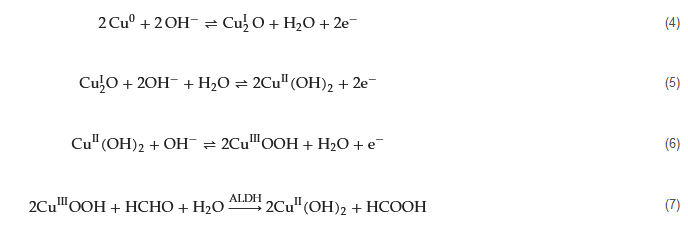
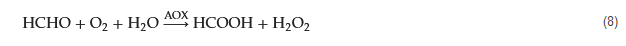
3. Electrochemical Sensors for Formaldehyde
3.1. Electrochemical Sensors Rely on Bioenzymes
3.1.1. Formaldehyde-Dehydrogenase-Based Formaldehyde Sensors
Formaldehyde dehydrogenase (FDH, EC 1.2.1.46) is an enzyme that plays a pivotal role in the metabolism of formaldehyde within living organisms. Its primary function involves catalyzing the oxidation of formaldehyde to formic acid, concurrently reducing the coenzyme nicotinamide adenine dinucleotide (NAD+) to NADH. This enzymatic reaction constitutes a vital component of the cellular pathway responsible for detoxifying formaldehyde.
Leveraging its enzymatic reactivity, FDH has been employed in the development of electrochemical biosensors tailored for formaldehyde detection. As far back as 1996, Hall and colleagues engineered sensors based on FDH for the direct measurement of formaldehyde vapor in gas-phase environments [60]. Within the electrochemical reaction cell, FDH is immobilized on the surface of either a platinum (Pt) or graphite working electrode. A gas-permeable Teflon membrane effectively segregates the gas phase (containing the target gas, formaldehyde) from the internal electrolyte of the electrochemical cell. Co-reactant NAD+ and electrochemical mediator 1,2-naphthoquinone-4-sulfonic acid (NQS) are introduced into the detection system. The detection mechanism hinges on the diffusion of formaldehyde vapor into the electrochemical cell. Here, FDH catalyzes the oxidation of formaldehyde while concurrently reducing NAD+ to NADH (Equation (1)). The electrochemical mediator NQS facilitates electron transfer between NADH and the electrode (Equations (2) and (3)). Notably, this sensor demonstrates a nearly linear response across formaldehyde concentrations ranging from 0 to 6 vppm, achieving a detection limit (LOD) of 0.3 vppm. Moreover, the sensor exhibits remarkable stability.
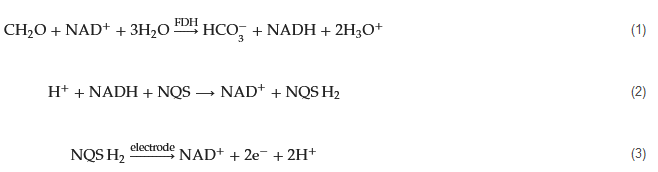
Researchers have developed a plethora of formaldehyde electrochemical sensors based on FDH by combining various electrode substrate materials, electrochemical electron transfer mediators, and different methods of enzyme immobilization on the electrode surface.
Metal and bimetallic nanoparticles have been widely employed to modify electrodes, significantly enhancing their surface area and catalytic activity. Such modifications are extensively used in electrochemical sensors to improve their performance. Ratautas et al. introduced the development of a direct electron transfer (DET) biosensor for formaldehyde determination in river water [75]. The biosensor involves immobilizing formaldehyde dehydrogenase (FDH) on a gold-nanoparticle-modified gold electrode.
Combining the traditional enzyme-based bioanode with various functionalized cathodes can create novel dual-mode responsive formaldehyde sensors. Dong and Zhai et al. introduced a novel self-powered biosensor (ESPB) for formaldehyde detection [39]. The key highlights and innovations of this sensor include the unique combination of components, including FDH/poly(methylene green) (PMG)/primary buckypaper (BP) as the bioanode and ferricyanide blue (PB)/gold nanoparticles (Au NPs)/carbon fiber paper (CFP) as the cathode. This innovative design enables self-powered formaldehyde detection without the need for an external power source. During operation, ESPB relies on the enzyme-catalyzed oxidation of formaldehyde occurring at the bioanode, generating NADH as fuel for the electrochemical reaction, thereby powering the device.
Copper exhibits a rich array of oxidation states, enabling various redox reactions to occur on the electrode’s surface, yielding different intermediates that facilitate electron exchange with other redox species. In their study, Song, Guo, and Liang et al. introduced an innovative and highly sensitive formaldehyde detection method based on the bioelectrocatalytic properties of ALDH (aldehyde dehydrogenases) and a copper electrode [35]. The reaction process is depicted by Equations (4)–(6). During the cyclic voltammetry anodic scan, the Cu electrode sequentially generates Cu2O, Cu(II), and Cu(III) oxide and hydroxide combinations. Upon the reverse scan (cathodic process), high-valence copper species are reduced, leading to the appearance of a cathodic peak around −0.2 V corresponding to the Cu(II)/Cu(I) transition. In the presence of formaldehyde and ALDH, an increased amount of Cu(II) is generated during the anodic scan (Equation (7)).
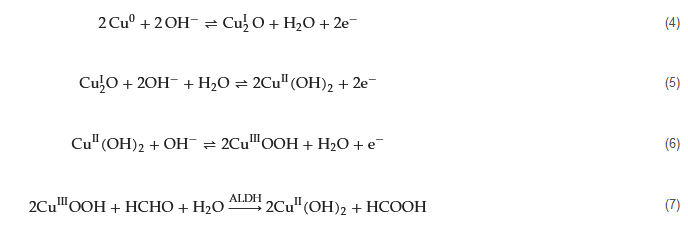
3.1.2. Alcohol-Oxidase-Based Formaldehyde Sensors
Alcohol oxidase (EC 1.1.3.13) is a crucial enzyme that catalyzes the oxidation of primary alcohols, including methanol. Studies have revealed that AOX can also catalyze the oxidation of formaldehyde to produce formic acid. AOX itself undergoes reduction as it accepts electrons from both methanol and formaldehyde. The reduced form of AOX is subsequently regenerated through oxidation by molecular oxygen (O2), leading to the generation of hydrogen peroxide (H2O2) as a byproduct. The overall reaction can be summarized as follows:
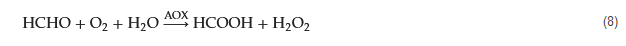
Therefore, AOX can be employed to fabricate electrochemical sensors for formaldehyde detection. Depending on the products and processes involved in the catalytic reaction, various types of electrochemical sensors can be developed. For instance, since formic acid can release hydrogen ions, a potential-based sensor with ion-selective membranes for hydrogen ion recognition can be constructed [78,81].
3.2. Electrochemical Sensors Rely on Electrocatalysts
3.2.1. Noble-Metal-Based Formaldehyde Sensors
Noble metal catalysts are important electrocatalysts, widely utilized in various fields such as oxygen reduction, hydrogen evolution, oxygen evolution, CO2 electroreduction, and oxidation of organic small molecules. Precious metal catalysts, including gold (Au), platinum (Pt), palladium (Pd), and silver (Ag), have also proven highly effective in the electrocatalytic oxidation of formaldehyde. They can efficiently and directly convert formaldehyde into carbon dioxide and water through different pathways, making them essential materials for constructing electrochemical formaldehyde sensors.
Au-based electrocatalysts
In as early as the year 2000, Hauser et al. devised a method for the direct amperometric detection of low concentrations of formaldehyde in the gas phase [84]. This method involved depositing gold onto a Nafion membrane as the working electrode. The study also explored the impact of gas flow rate and gas stream humidity on the sensor’s performance, along with its sensitivity to various organic and inorganic gases. Furthermore, the researchers proposed a solution to counter interference from NO, NO2, and SO2 by selectively adsorbing formaldehyde from the sample stream using an aluminum oxide filter. This approach boasts a low detection limit and an extensive dynamic range, making it highly suitable for continuous air monitoring applications. Subsequently, Surareungchai et al. achieved the sensing of formaldehyde using an unmodified gold electrode through the application of pulsed amperometric detection (PAD) in a flow injection (FI) system [85].
In recent years, there have been studies employing various methods to prepare nanostructured gold materials with precise structural features, aiming to enhance their catalytic performance. Kumar et al. utilized an in situ electrochemical process to fabricate gold (111)-oriented nanoparticles on the surface of carbon nanofibers–chitosan composite (GCE/CNF-CHIT@Aunano) [87]. This innovative approach resulted in the development of a sensor capable of detecting formaldehyde in buffered solutions. Through a range of physicochemical characterization techniques, it was revealed that amino-functionalized chitosan stabilizes gold (111) nanoparticles with a size of approximately 10 nm within the composite matrix. In comparison to other catalysts, these Au(111) catalysts exhibit exceptional catalytic activity, stability, and remarkable surface characteristics. The mechanism of formaldehyde electrocatalytic oxidation on the GCE/CNF-CHIT@Aunano modified electrode was investigated using cyclic voltammetry (CV) and electrochemical quartz crystal microbalance (EQCM). During the anodic scan, formaldehyde exhibited oxidation onset at 0.15 V, followed by a broad anodic peak at 0.4 V. In the reverse scan, a well-defined anodic peak was observed at the same potential. The mechanistic pathway for formaldehyde oxidation involves two possible routes: a direct pathway (dehydrogenation) and an indirect pathway (dehydration) via the adsorption of CO as a poisoning species.
Deposition of gold nanoparticles onto polymer surfaces has proven to be an effective technique, allowing for the efficient binding and dispersion of nanoparticles due to the functional groups present on the polymer surface. Huang et al. were the first to modify a glassy carbon electrode (GCE) with polypyrrole (PPy) and subsequently electrodeposit gold nanoparticles (AuNPs), resulting in a functional electrode denoted as GCE/PPy/AuNPs [88]. This electrode exhibited high electrical conductivity and excellent catalytic activity, enabling the sensitive quantification of formaldehyde. Liu et al. presented the development of a molecularly imprinted electrochemical sensing platform tailored for the specific detection of formaldehyde [89]. They immobilized gold nanoclusters (Au NCs) onto the surface of polydopamine nanospheres (pDA NPs) to create a pDA@Au NCs composite material. Subsequently, molecularly imprinted polymers (MIPs) for formaldehyde were synthesized on the surface of pDA@Au NCs, yielding the pDA@Au NCs-MIPs-HOPG (highly oriented pyrolytic graphite) electrode. This sensor, combining molecular imprinting technology (MIT) with the catalytic properties of noble metal nanoparticles (Au NCs), demonstrated impressive selectivity and high sensitivity. It featured a broad detection range spanning from 0.2 μM to 0.02 M and a low detection limit of 0.1 μM. The sensor was effectively applied to detect trace amounts of formaldehyde residues in seafood, particularly octopuses, showcasing satisfactory selectivity and reproducibility.
Pt-based electrocatalysts
Similar to gold nanoparticles, platinum nanoparticles also possess high specific surface area, excellent electrocatalytic activity, and stability. They have significant potential for use as electrocatalysts in constructing formaldehyde electrochemical sensors. Platinum nanoparticles can promote the oxidation reaction of formaldehyde, thereby enhancing the current response and enabling rapid and accurate detection of formaldehyde concentrations. Furthermore, the nanoscale size and tunable structure of these materials provide ample room for further optimizing sensor performance, including improving selectivity and reducing interference.
Zen et al. developed an innovative electrochemical sensor for formaldehyde gas [92], featuring a platinum-coated screen-printed ultramicroelectrode wrapped in Nafion as the electrolyte. This sensor’s novelty stems from its detection mechanism, which uses high oxidation potential to transform formaldehyde into formic acid, thereby activating Pt catalyst sites. Employing square wave voltammetry, the sensor distinctly separates responses from platinum oxide reduction and formic acid oxidation, achieving a broad linear detection range with an impressive sensitivity down to 80 ppb.
Pd-based electrocatalysts
Palladium (Pd) nanomaterials exhibit high catalytic activity in the electro-oxidation of organic small molecules. Furthermore, compared to Pt, Pd-containing materials are particularly attractive due to their relatively lower cost and high tolerance to CO formation as a byproduct during formaldehyde oxidation. The use of nanoparticles with high electroactive surface areas can enhance the sensitivity of these materials to formaldehyde oxidation. Yi et al. employed a hydrothermal method, using PdCl2, EDTA (ethylenediaminetetraacetic acid), and formaldehyde as precursors, to prepare unique three-dimensional porous Pd nanoparticles on a titanium (Ti) substrate [96]. These nanostructured Pd electrodes exhibited outstanding electrocatalytic performance for formaldehyde oxidation in alkaline solutions. They demonstrated a low onset potential for formaldehyde electro-oxidation on the nanoPd electrode, approximately −0.85 V vs. the saturated calomel electrode (SCE), along with a significantly large anodic current density of 66.96 mA·cm2.
Ag-based electrocatalysts
Nanoporous silver, owing to its high electrical conductivity, large surface area, high porosity, and cost-effectiveness, finds extensive applications in catalyzing the oxidation of small organic molecules. The catalytic activity of nanoporous silver catalysts is closely linked to their size, morphology, structure, and the physical environment in which they operate. Jeon et al. reported the synthesis of silver nanoparticles (Ag NPs) on graphene oxide functionalized with p-phenylenediamine (Px), resulting in the formation of GOPx-Ag nanocomposites [106]. These nanocomposites were employed as catalysts for the direct electro-oxidation of formaldehyde, with an onset oxidation potential of −0.783 V. Cyclic voltammetry tests revealed oxidation–reduction processes (Ag↔Ag2O) occurring on the electrode surface of Ag. In comparison to electrodes lacking Px, GOPx-Ag-modified electrodes exhibited a 57.3% increase in the oxidation response current for formaldehyde and a 159 mV negative shift in oxidation potential. This indicates that Px enhances the dispersion of Ag NPs, leading to higher electrocatalytic activity. The authors also explained that Px-functionalized graphene oxide provides N lone pair electrons. This functionalized GO is a dense two-dimensional (2D) material with unparalleled electrical conductivity.
3.2.2. Bimetallic-Based Formaldehyde Sensors
Bimetallic nanocatalysts have demonstrated significant advantages in catalyzing the oxidation of formaldehyde due to their unique properties. These catalysts typically consist of two different metals, leading to synergistic effects that enhance catalytic efficiency, selectivity, and stability. They often exhibit superior activity compared to single-metal catalysts, possibly due to improved electron transfer, increased active sites, and altered reaction pathways. Recent research has focused on optimizing the composition, structure, and size of bimetallic nanoparticles to further enhance their performance in formaldehyde oxidation. These advancements include the development of novel synthesis methods, understanding the nanoscale interaction between the two metals, and exploring various combinations of metals. Currently reported bimetallic nanocatalysts for electrochemical detection of formaldehyde include Pt-Ru [109], Pd–Pt [110,115,156], Pd-Au [111], Ag-Pd [112], Sn-Pt [113], Cu-Pd [114,116], Ni-Pd [117], Pt–Ag [118], and Cr-Pd [119]. Due to Pd’s high catalytic activity and low susceptibility to poisoning during anodic oxidation, it is commonly used in alloy materials. Bimetallic catalysts comprising Pd and other noble metals have shown excellent catalytic performance in formaldehyde electro-oxidation.
Mardared et al. investigated the electrocatalytic performance of various compositions of copper–palladium (CuPd) thin film combinatorial libraries using cyclic voltammetry throughout the entire composition range of CuPd films [114]. They found that Cu-7.5 at.% Pd exhibited the highest electrocatalytic activity for formaldehyde oxidation with a starting potential of −0.35 V and a current density of 1.81 mA∙cm−2. The observed electrocatalytic enhancement at the optimal composition was attributed to the synergistic effects involving Pd concentration, surface properties, and electron density. Azizi developed a highly sensitive electrochemical sensor for formaldehyde detection using novel bimetallic nanoporous Pd-Cu-SBA-16/CPE (carbon paste electrode) [116]. Bimetallic nanoparticles composed of palladium (Pd) and copper (Cu) were incorporated into SBA-16 using an electrochemical replacement reaction. This approach reduced the use of precious metal (Pd) while improving electrocatalytic performance. The electrochemical properties of the Pd-Cu-SBA-16/CPE formaldehyde oxidation sensor were thoroughly investigated using cyclic voltammetry, current analysis, and chronoamperometry. The sensor exhibited excellent electrocatalytic activity with high current density and low formaldehyde oxidation overpotential. Wang and Li et al. developed an electrochemical method for on-site detection of formaldehyde in food using Pt-Ag core–shell nanoparticles as the electrocatalyst [118].
Metallenes, a cutting-edge topic in the field of materials science, have emerged as a novel class of two-dimensional materials composed of single layers of metal atoms, exhibiting unique physical and chemical properties [157,158,159]. In the realm of electrocatalysis, metallenes hold tremendous potential owing to their high surface area, excellent electrical conductivity, and distinctive electronic structure [160,161,162]. These attributes render them promising candidates for various applications, including catalyst supports [163,164], energy storage [165,166], and sensors [167,168].
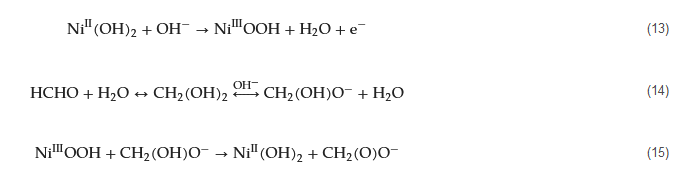
3.2.3. Transition Metals and Their Oxide-Based Formaldehyde Sensors
Transition metal materials have shown significant potential in electrochemical catalysis for formaldehyde oxidation. These materials, including nickel, copper, and other metal oxides, are capable of undergoing various oxidation states, which facilitates effective electron mediation required for formaldehyde oxidation. These catalytic materials can be obtained through various preparation methods, such as electrochemical deposition, vapor-phase deposition, chemical corrosion, in situ transformation, and more. They exhibit high catalytic activity for the electrochemical oxidation of formaldehyde and hold great promise in the construction of formaldehyde sensors. The following discussion will categorize these materials and their applications.
Nickel-based electrocatalysts
Nickel-based transition metal nanomaterials are the most widely used electrocatalysts for constructing electrochemical formaldehyde sensors. They are typically prepared using methods such as electrochemical deposition and chemical corrosion on different substrates. The mechanism of formaldehyde oxidation catalysis by these catalysts is well understood and often involves the conversion of Ni(II) to Ni(III) (Equation (13)). Subsequently, Ni(III)OOH species oxidize the ionized form of formaldehyde, CH2(OH)O− (Equations (14) and (15)), and Ni returns to its initial divalent state as Ni(OH)2.
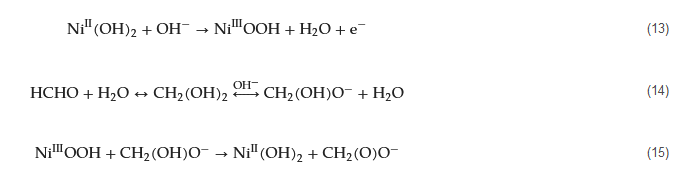
Ying et al. have introduced a sensitive and cost-effective flow injection analysis (FIA) method for detecting formaldehyde using an activated barrel-plated nickel electrode (Ni-BPE) [120]. The mechanism of formaldehyde electrocatalytic oxidation on the Ni-BPE electrode in an alkaline medium at ambient temperature is discussed, involving the oxidation of formaldehyde by NiIIIO(OH) species. This method exhibits good linearity within the concentration range of 0.037 to 10 μg∙mL of formaldehyde, with a low LOD of 0.23 μg∙L. The method demonstrates excellent reproducibility and has been successfully applied to the determination of formaldehyde in commercial nail polish samples and drinking water.
Copper-based electrocatalysts
Copper exhibits various oxidation states, including Cu, Cu(I), Cu(II), and Cu(III), during the electrochemical process, which can act as effective electron-conducting intermediates for the oxidation of formaldehyde. Consequently, copper is widely employed in the construction of formaldehyde electrochemical sensors. Abnosi et al. investigated the electrocatalytic oxidation behavior of formaldehyde on a copper electrode in alkaline solutions [135]. Their cyclic voltammetry studies revealed that the presence of formaldehyde resulted in an increase in peak current associated with Cu(III) oxidation, followed by a corresponding decrease in cathodic current.
Other studies have suggested that Cu(II) also plays a crucial role as a mediator in the oxidation of formaldehyde. Farhadi et al. prepared copper-porous silicon nanocomposite materials (Cu/PS) and explored their application in the detection of formaldehyde in electrochemical sensing [139]. They first deposited copper nanoparticles onto etched porous silicon (PS) surfaces using an electrodeposition method, resulting in Cu/PS nanocomposite materials. Cu NPs/PS/SPCE exhibited significant electrocatalytic activity for the oxidation of formaldehyde at negative potentials. Compared to an unmodified electrode, the peak potential shifted approximately 0.7 V in the negative direction. Electrochemical tests suggested that the oxidation peak on Cu NPs/PS/SPCE was mainly associated with direct formaldehyde oxidation. Cu(II) ions generated during the forward scan on Cu NPs/PS/SPCE could effectively serve as intermediates for formaldehyde oxidation, resulting in a substantial increase in oxidation peak current. Tang et al. investigated the fabrication of a CuO/Cu/TiO2 nanotube array (TNA)-modified electrode and its performance in formaldehyde detection [141]. The CuO/Cu/TNA-modified electrode, crafted through an electrochemical approach, showed remarkable performance in catalyzing formaldehyde’s electrocatalytic oxidation.
Other transition metal oxides or sulfides, such as TiO2/RuO2 [144], MnO2 [145], CeO2 [149], Co(OH)2 [147], MoOx [146], ZnO [150,151], and Ag2S [148], have also demonstrated the ability to catalyze the oxidation of formaldehyde. Bertazzoli et al. investigated the kinetics of formaldehyde oxidation in a flow electrochemical reactor with a TiO2/RuO2 anode [144]. The reactor employed a titanium electrode coated with (TiO2)0.7(RuO2)0.3 and monitored the electrochemical degradation of formaldehyde solutions. The oxidation of formaldehyde, as well as the removal of total organic carbon (TOC) and chemical oxygen demand (COD), was primarily controlled by mass transfer. For a solution containing 0.4 g∙L−1 of formaldehyde, the electrochemical degradation followed pseudo-first-order kinetics. The TiO2/RuO2 anode combination exhibited a higher rate of formaldehyde and formic acid oxidation compared to electrodes containing IrO2. Nakayama et al. electrochemically deposited MnO2 thin films onto glassy carbon electrodes using cathodic reduction from a KMnO4 solution [145]. The MnO2 was of the hollandite-type structure and demonstrated catalytic oxidation of formaldehyde under mild pH conditions (pH 6.3 or 4.0).
3.2.4. Organic-Polymer-Electrocatalysts-Based Formaldehyde Sensors
Organic polymer electrocatalysts, typically formed by covalent bonds in organic structures, feature large conjugated systems, providing excellent electrical conductivity and stability [176,177,178,179]. These cost-effective polymers are easily shaped and made through electrochemical polymerization or chemical redox methods. This process forms active sites on their surface or interior, enhancing their catalytic activity, particularly in oxidizing formaldehyde. Notable examples include polypyrrole [152], polyaniline [155], polydopamine [153], and polyacrylonitrile [154]. Advancements in synthesis and the exploration of new materials are expected to further improve these catalysts’ efficiency and broaden their applications.
3.3. Electrochemical Sensors Rely on Derivative Reagents
Formaldehyde, as an active organic molecule, exhibits specificity in its reactions with various organic functional groups, primarily due to its electrophilic nature and the presence of its carbonyl group. These specific reactions include the Lindlar reaction, which involves the formation of Schiff bases through the reaction of formaldehyde with amino compounds, the 2-aza-Cope reaction that leads to the rearrangement of imine structures, aldol reactions where formaldehyde adds to other carbonyl groups, and amine–formaldehyde reactions that result in the generation of compounds like urea. Leveraging these distinctive reactions, especially when combined with appropriate derivatization reagents, enables the development of highly selective formaldehyde electrochemical sensors.
Earlier research demonstrated that formaldehyde reacts with acetylacetone and ammonia to form a yellow substance, 3,5-diacetyl-1,4-dihydromethylpyridine (DDL), a reaction historically utilized for formaldehyde detection via spectrophotometry. In a novel approach, Silva found that DDL undergoes oxidation on unaltered glassy carbon electrodes, with an oxidation peak at 0.8 V, where formaldehyde is not electroactive (Figure 14) [58]. This discovery suggests the potential for indirectly and selectively detecting formaldehyde electrochemically. The method showed a linear detection range from 0.4 to 40.0 mg∙L−1 and a low detection limit of 0.13 mg∙L−1, proving especially useful for quickly identifying formaldehyde in diverse samples.
4. Conclusions
(1) Diversity in Sensing Mechanisms: electrochemical sensors for formaldehyde detection are developed based on diverse principles like enzymatic reactions, usage of electrocatalysts, and specific chemical reactions, each offering unique advantages in terms of sensitivity, selectivity, and scope of application.
(2) Enzymatic Sensors: Primarily utilizing FDH, these sensors display high specificity and sensitivity towards formaldehyde. Advances in electrode material engineering and enzyme immobilization have notably enhanced their performance.
(3) Electrocatalyst-Based Sensors: Employing metals, metal oxides, and bimetallic nanocatalysts, these sensors are promising due to their high electrocatalytic activity and stability. Innovations in nanostructuring and surface modification have improved sensitivity and selectivity in formaldehyde detection.
(4) Chemical-Reaction-Based Sensors: Utilizing formaldehyde’s specific chemical reactivity, these sensors offer a highly selective detection method. Success depends on the precise selection of derivatization reagents and understanding underlying chemical interactions.
This entry is adapted from the peer-reviewed paper 10.3390/molecules29020327
This entry is offline, you can click here to edit this entry!
