Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Thoracic aortic aneurysm (TAA) has a prevalence of 0.16–0.34% and an incidence of 7.6 per 100,000 person-years, accounting for 1–2% of all deaths in Western countries. No effective pharmacological therapies have been identified to slow TAA development and prevent TAA rupture. Large TAAs are treated with open surgical repair and less invasive thoracic endovascular aortic repair, both of which have high perioperative mortality risk. Therefore, there is an urgent medical need to identify the cellular and molecular mechanisms underlying TAA development and rupture to develop new therapies.
- Marfan syndrome
- β-aminopropionitrile
- calcium chloride
- elastase
- angiotensin II
1. Introduction
Thoracic aortic aneurysms (TAAs) refer to a localized thoracic aortic dilatation of greater than 50% of the normal diameter. TAAs can occur in the ascending aorta, the aortic arch, and the descending thoracic aorta, with some aneurysms involving multiple segments. The majority of TAAs involve the ascending thoracic aorta (60%), followed by the descending aorta (40%) and the aortic arch (10%) [1,2]. TAAs have an estimated prevalence of 0.16–0.34% and an incidence of 7.6 per 100,000 person-years [3]. TAAs account for 1–2% of all deaths in Western countries [4].
Risk factors for TAA development include male sex, older age, smoking, hypertension, chronic obstructive pulmonary disease, coronary artery disease, previous aortic dissection, and a family history of TAA and TAA-related disorders including bicuspid aortic valves, Ehlers–Danlos syndrome, Loeys–Dietz syndrome, Marfan syndrome, and Turner syndrome [5,6]. Atherosclerosis may be a risk factor for TAA [7]. It has been reported that atherosclerosis negatively affects the biomechanical properties of the descending thoracic aorta and decreases aortic resistance to tearing [8]. The decrease in aortic resistance to tearing strongly correlates with the presence of calcification [8]. It is worth noting that a recent study shows that TAA is not associated with atherosclerotic burden [9]. In addition, statin use is not associated with long-term mortality in patients with TAA undergoing endovascular repair [10].
There are two aortic repair-type interventions to treat large TAAs: open surgical repair and thoracic endovascular aortic repair (TEVAR) [11]. For treating TAAs in the descending thoracic aorta, open surgical repair is associated with a high perioperative mortality risk ranging from 2.7–8%, and the risk for TEVAR ranges from 2.1% to 6.1% [11,12,13]. Meta-analyses of non-randomized comparison studies have shown that early mortality is lower after TEVAR than open surgical repair [11,12,13,14,15]; however, overall mid-term survival (≥1 year) does not differ between these two treatment options [12,14]. In addition, endoleaks occur in 8.1% to 19.5% of cases after TEVAR [14,15,16]. In patients involving the ascending aorta (e.g., those with Marfan syndrome), open surgical repair should be preferred over TEVAR [17,18] as TEVAR is associated with a higher mortality rate of 15.2% [19].
Small TAAs are monitored by repeated computed tomography (CT) or magnetic resonance imaging (MRI). Once the 5.5 cm threshold is reached, patients without risk factors for dissection will be referred to aortic repair procedures [1,20].
Patients with TAAs are recommended to lower blood pressure using β-blockers, angiotensin-converting enzyme inhibitors, and angiotensin receptor blockers, to potentially reduce rupture and dissection risk [1]. However, no effective pharmacological therapies have been identified to slow TAA development and prevent rupture.
Considering the potential limitations of open surgical repair and TEVAR and the lack of effective pharmacological therapies for TAA, it is a major medical need to understand the cellular and molecular mechanisms underlying TAA pathogenesis better and to develop new therapies including those to prevent endoleak following TEVAR.
2. Pathological Features of Human TAAs
The aorta is composed of three layers: the tunica intima, tunica media, and tunica adventitia. The tunica intima is composed of one layer of endothelial cells attached to the basal lamina. The tunica media is composed of more than 50 alternating layers of vascular smooth muscle cells (VSMCs) and elastic fibers. The tunica adventitia is composed of fibroblasts, loose connective tissue, and vasa vasorum [21]. The major pathological features of human TAA include endothelial dysfunction, elastin fragmentation, loss of VSMCs via increased apoptosis, increased deposition of proteoglycans in the tunica media, and excessive accumulation of collagen (vascular fibrosis) [21,22,23,24]. These changes are often accompanied by an increase in inflammation, oxidative stress, and matrix metalloproteinases (MMPs) [23].
TAAs become progressively larger and this enlargement enhances the risk of aortic dissection and rupture [21]. Thoracic aortic dissections develop when a tear in the tunica intima occurs, which allows blood to flow into the aortic wall to form a false lumen.
3. Rodent TAA Models
Rodents are the most commonly used animals in TAA research and they have contributed greatly to our understanding of the pathogenesis of this disease. TAAs in rodents can be induced by various methods including chemicals, surgery, and genetic manipulation.
3.1. β-Aminopropionitrile (BAPN)-Induced TAA in Rodents
BAPN is the most commonly used chemical to induce TAAs in rodents [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. BAPN is an inhibitor of lysyl oxidase, and the latter mediates the crosslinking process of elastin and collagen. Therefore, BAPN treatment weakens the arterial wall and promotes aneurysm formation [44,45].
Often, 3-week-old male C57BL/6 mice are used in this model. Mice are treated with BAPN (1 g/kg body weight per day) via drinking water for 4 weeks to induce TAAs. There are some variations in this model, including genetic background, age, and sex. For example, C57BL/10 mice [34], mice aged 4 weeks [41], and mice of both sexes [37] have been used. In addition, lower doses of BAPN (e.g., 0.5 g/kg body weight per day [34,35,36]) have been employed. Moreover, the induction time can be shortened to 1 or 2 weeks to observe early changes in TAA initiation [37] or it can be extended up to 26 weeks to observe late changes in disease progression including TAA rupture and mortality [41]. It is worth noting that rats are also used in this model [43].
TAAs induced by BAPN show thrombosis, degradation of elastic fibers, and loss of VSMCs [46], bearing similarities to human TAAs. In addition, this rodent model shows thoracic aortic dissection and therefore it can be used to investigate this TAA-associated complication.
3.2. Angiotensin II Infusion-Induced TAA in Rodents
Angiotensin II is a commonly used TAA-inducer [47,48,49,50,51,52,53]. In this model, apolipoprotein E-deficient mice are often employed [47,48,49]. To induce TAAs, mice are subcutaneously infused with angiotensin II at a dose of 1 μg/kg body weight per minute using an osmotic pump. Occasionally, higher doses of angiotensin II (e.g., 1.5 μg/kg body weight per minute [51]) are used. Angiotensin II-infused wild-type mice serve as controls to examine the impact of a certain gene on TAA formation [50,51,52]. Moreover, rats can be used in this model [53]. It is worth noting that angiotensin II infusion also induces aortic aneurysms in the abdominal region [54,55], and therefore, this model is a model of thoracoabdominal aortic aneurysms [56,57]. Finally, this model is also suitable for investigating TAA rupture and dissection.
3.3. Elastase-Induced TAA in Rodents
Elastase is another common TAA-inducer [58,59,60,61]. Elastase is applied to the surface of the aorta using a presoaked sponge for a certain time [58,59,60,61]. Elastase can be applied to the ascending aorta and aortic arch [58] or the descending thoracic aorta [59,60,61]. Both adult mice [58,59,60] and rats [61] have been studied for this purpose. The elastase dose and application time vary and a dose-response curve is recommended for each new bottle of elastase as the digestive power of elastase differs from bottle to bottle [60]. An optimal application time would create an aortic expansion of 100–130% at the end of the experiment [60]. Longer elastase application time leads to TAA rupture [58].
An advantage of this model is that TAAs are formed in a short time window, often 2 weeks. In addition, the TAA location can be controlled. This model has some disadvantages. First, it may not closely represent human TAA development which requires years or decades. Second, elastase-induced aneurysms begin to decrease in size when they reach maximal dilatation at two weeks post-surgery, which is different from human TAAs that become progressively larger [60].
3.4. Calcium Chloride-Induced TAA in Rodents
Calcium chloride is also a common TAA-inducer [62,63,64,65]. In this model, the descending thoracic aorta is treated perivascularly with calcium chloride (0.5 M) for 15 min with a presoaked gauze applicator [62,63,64,65]. This method has been used for both mice [62,63] and rats [64,65]. This animal model represents some features of human TAAs including an increase in apoptosis [64,65], inflammation [65], and extracellular matrix degradation [62,63].
However, this method usually generates a smaller increase in aortic diameter of about 18–25% [62,64], although a larger increase in aortic diameter (~60%) has also been reported [63]. Calcium phosphate treatment can increase the abdominal aortic diameter to a larger extent (95%) [66,67], and whether calcium phosphate is superior to calcium chloride for TAA induction remains to be investigated. It is worth noting that the TAA size does not further expand from week 4 to week 16 after the calcium chloride application. Therefore, this model is not suitable for investigating TAA progression and rupture.
3.5. Combination of BAPN and Angiotensin II-Induced TAA in Rodents
The combination of BAPN and angiotensin II has been reported to induce TAA [46,68,69,70]. Often 3-week-old male C57BL/6 mice are used. Mice are treated with BAPN for 4 weeks followed by 1–3 days of angiotensin II infusion (1 μg/kg body weight per minute) to induce TAAs [46,68,70]. BAPN can be administrated via intraperitoneal injection [68], drinking water [69,70], or as a diet supplement [46]. Angiotensin II infusion can be extended to 28 days which increases the percentage of mice that develop TAA [68]. Angiotensin II infusion can also be administrated at the same time as BAPN [69]. This animal model shows a sex phenotype with female mice showing a smaller aortic expansion and less medial degradation compared with their male counterparts [69]. Moreover, aortic dissection may be studied [46]. Similar to human TAAs, the TAAs in these mice show intramural hematomas, elastic fiber degradation, and inflammatory cell infiltration [69].
3.6. Combination of High-Fat Diet and Angiotensin II-Induced TAA in Rodents
TAAs can be induced in C57BL/6 mice by feeding the animals with a high-fat diet for 8 weeks followed by subcutaneous infusion of angiotensin II (2 μg/kg body weight per minute) during the last 4 weeks [71]. The approach is suitable to study TAA-associated dissection, as more than half of the animals develop the phenotype [71].
3.7. Transverse Aortic Constriction-Induced TAA in Rodents
Transverse aortic constriction induces aortic dilation due to pressure overload. To perform this procedure, a silk suture (size: 6–0 or 7–0) is tied around a 27-gauge needle overlying the arch at a location between the brachiocephalic trunk and the left common carotid artery [40] (Figure 1). Then, the needle is promptly removed which yields a constriction of approximately 0.3 mm as the outer diameter of the 27-gauge needle. This produces aortic constriction of 60–80% [72].
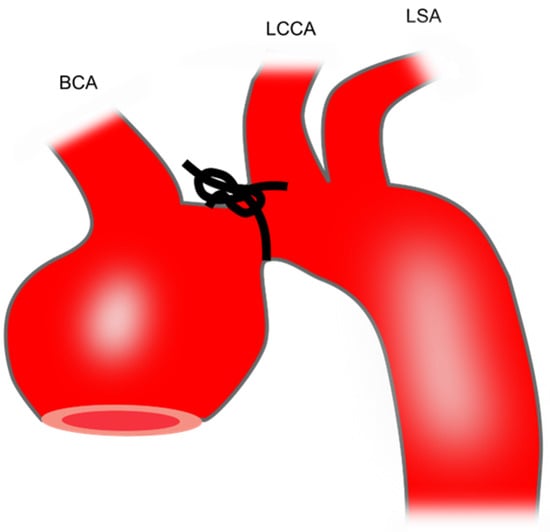
Figure 1. Preparation of mouse TAA using transverse aortic constriction. The black knot shows the area of constriction. BCA, brachiocephalic artery; LCCA, left common carotid artery; LSA, left subclavian artery; TAA, thoracic aortic aneurysm.
This model leads to an increase in ascending aortic diameter by 21–23% after 1 [73], 2 [72], or 3 weeks [40]. Therefore, this model is not technically classified as a TAA model which requires an increase in aortic diameter of at least 50%. Whether the aortic diameter further increases with longer follow-up requires future study.
3.8. Genetic TAA Models in Rodents
Marfan syndrome affects 1 in 5000 individuals worldwide [74] and TAA (in particular at the aortic root and ascending aorta) is one of its clinical presentations. The disease is most commonly caused by a variant in the Fbn1 gene which codes the fibrillin-1 protein. Fibrillin-1 interacts with elastin to provide strength and elasticity to blood vessels. Fibulin-4 (coded by the Fbln4 gene) is important for elastogenesis. Therefore, defects in fibrillin-1 or fibulin-4 weaken the aortic structure and lead to TAA formation. The most commonly used genetic models of TAA are mice deficient in Fbn1 or Fbln4. In addition, mice deficient in transforming growth factor-beta (TGFβ) receptor 2 are often used as a TAA model.
Fbn1C1041G/+ mice: This mouse model of Marfan syndrome represents the most commonly used genetic model in investigating TAA [40,52,75,76,77,78,79,80,81,82,83,84,85,86,87]. Fbn1C1041G/+ mice, also known as Fbn1C1039G/+ mice, are generated by substitution of cysteine with glycine at amino acid 1041 (C1041G) in exon 25 of fibrillin-1 (previously identified in the literature as C1039G) [88]. This missense mutation presents in a subgroup of patients with Marfan syndrome [88]. These mice show a decreased deposition of microfibrils. The aorta of the Fbn1C1041G/+ mice starts to deteriorate after 2 months, showing overexpression of MMP-2 and -9, elastic fiber fragmentation, disarray of VSMCs, and increased collagen deposition [88]. Inflammatory cell infiltration is not prominent in this model. Fbn1C1041G/+ mice exhibit moderate TAA without dissection [88], have a normal lifespan [88], and show sex dependency, i.e., TAAs present dominantly in male mice [76,82].
Fbn1mgR/mgR mice: These mice are another model of Marfan syndrome and are the second most commonly used genetic model in TAA [49,89,90,91,92,93,94,95,96]. The Fbn1 mgR allele is generated by insertion of the PGKneo expression cassette into an intron region of the Fbn1 gene without loss of the Fbn1 exon sequence [97]. The resultant mgR protein has the same size as wild-type fibrillin-1. Fbn1mgR/mgR mice have reduced mgR expression of approximately 20% of the normal amount. These Fbn1 hypomorphic mice rapidly develop ascending aortic aneurysms with macrophage infiltration, calcified tunica media, and elastic fiber fragmentation [97]. TAAs progress fast and dissection is fully penetrant in these mice. Therefore, this mouse line allows to investigate the progression of TAA-associated dissection and survival [97]. Fbn1mgR/mgR mice represent a progressively severe model of Marfan syndrome and most of the affected animals die from dissecting TAAs within 3 months after birth [97,98].
Fbln4SMKO mice: Mice lacking fibulin-4 in smooth muscle cells (Fbln4SMKO) are another genetic model of TAA [49,99,100,101,102,103]. Animals die spontaneously when they are approximately 2 months old. Mice develop large aneurysms exclusively in the ascending aorta which is associated with a VSMC differentiation defect and focal hyperproliferation of VSMCs [104].
Fbln4R/R mice: The fibulin-4 R allele is generated by inserting a neomycin resistance gene-expressing cassette into the fibulin-4 gene, which leads to a 4-fold decrease in fibulin-4 expression through transcriptional interference [105]. All newborn mice develop TAAs at the ascending aorta resulting from disorganized elastic fiber networks. These mice start to die after 9 days.
Fbln4E57K/E57K mice: The knock-in mutant mice are generated by substitution of glutamic acid with lysine at amino acid 57 (E57K) in exon 4 of the Fbln-4 gene. The mutant fibulin-4 protein is prone to dimerization and is ineffectively secreted. Homozygous Fbln4E57K/E57K mice can survive to 1 year of age and develop large TAA at the aortic root and ascending aorta (at least 2-fold increase in diameter compared with wild-type mice) in ~50% of the mice by 7 months [106,107].
Tgfbr2SMKO mice: These mice represent a conditional deletion of TGFβ receptor 2, specifically in smooth muscle cells. Tgfbr2SMKO mice form dissecting TAAs in the ascending aorta and the aortic arch. Aortas show increased inflammation, elastic fiber fragmentation [108], and mural hematomas [109]. Tgfbr2SMKO mice can cross-breed with Fbn1C1041G/+ to generate compound mutant mice (Tgfbr2SMKO/Fbn1C1041G/+ mice) that have faster and more severe TAA growth compared with Fbn1C1041G/+ mice [109].
Other genetically modified mice can spontaneously develop TAAs and could be potentially used as genetic TAA models. For example, α-L-iduronidase-deficient mice (Idua−/− mice) display progressive accumulation of glycosaminoglycans in the aorta [110] and develop TAAs which are more severe in males than females [111,112,113]. Fbn1GT−8/+ mice, expressing a truncated fibrillin-1 protein display limited dilatation (<50%) of the thoracic aorta at the age of 8–12 months [114].
4. Porcine TAA Models
Although rodent models are very important experimental tools, there are differences between rodents and humans in metabolism, anatomy, and physiology [115]. In contrast, pigs better resemble human anatomy and physiology. For example, pigs and humans have similar heart rate and blood pressure [116,117,118,119,120].
Porcine TAA models have other advantages. They enable evaluation of new treatments for TAA by angiographic imaging, resembling clinical settings. In contrast, rodent models of TAAs are not suitable to be monitored using angiographic imaging. In addition, these porcine models could be used to assess the effectiveness of new therapeutic devices or interventions intended for clinical use in humans, in particular, to identify treatments for endoleaks after TEVAR [121,122] and new methods to minimize the damage caused by open surgical repair [123]. In contrast, rodent models are not suitable for testing TAA surgical repair procedures or devices due to the small size of the animals.
One common limitation of using pig models, however, is that there is no reliable method to assess anesthetic depth during surgery [124].
4.1. Intra-Adventitial Injection of Elastase
Following thoracotomy, a 4 cm thoracic descending aortic segment proximal to the left subclavian artery is isolated. Elastase (a total of 5 mL, 20 mg/mL) is circumferentially injected into the adventitia of the isolated segment of the aortic wall starting from 0.5 cm away from the left subclavian artery and expanding 2 cm distally toward the diaphragm [18]. Twelve injection points are distributed in this 2 cm aortic segment with each point being injected with 0.4 mL of elastase [18]. This model is characterized by a loss of VSMCs and degradation of elastic fibers [18].
4.2. Intra-Adventitial Injections of Collagenase in Combination with Periadventitial Application of Calcium Chloride
Following thoracotomy, the descending thoracic aorta is dissected from the surrounding tissue to create a ~5 cm area [125]. Collagenase (5 mL, 0.35 mg/mL in saline with 0.1 mol/L calcium chloride) is circumferentially injected into the tunica adventitia of the isolated region. A piece of absorbable gelatin sponge is placed under the aortic region and 0.5 g of calcium chloride powder is then applied periadventitially to the isolated aortic segment. A gel foam is then wrapped around the aorta to enclose the area of calcium chloride application and to minimize irritation to the lungs and heart.
Three weeks after TAA induction, the aorta dilatates to 38 ± 13% without rupture. The aortas of these pigs show an increase in fibroblasts, an increase in MMPs, and a decrease in VSMCs [125].
4.3. Vein Patch Method
This vein patch method requires an invasive step for harvesting patch materials [16]. The left jugular vein is harvested with the maximum possible length and cut open longitudinally, and the two short ends are then sewn together (Figure 2). After thoracotomy, the exposed thoracic aorta is dissected from the surrounding tissue. The aorta is side-clamped and incised longitudinally for approximately three quarters of its diameter. The vein graft is then sutured to the aorta and the top line of the vein graft is sewn (Figure 2).
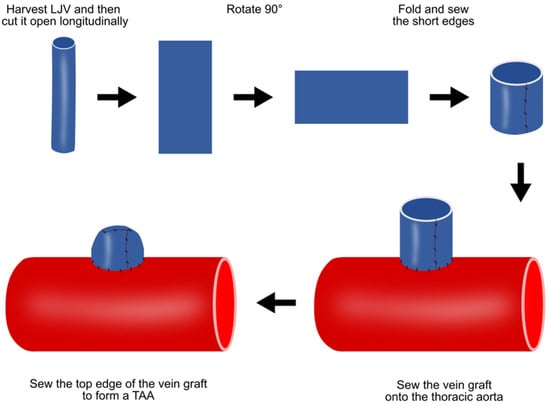
Figure 2. Preparation of porcine TAA using the vein patch method. LJV, left jugular vein; TAA, thoracic aortic aneurysm.
This method has several limitations. TAAs formed via this method are histologically different from real aneurysms. In addition, TAAs are saccular, not in a fusion form. However, this TAA model is expected to be valuable in developing novel treatments for endoleaks after TEVAR.
4.4. Pericardium Pouch Method
The commercially available bovine pericardium patch is treated with glutaraldehyde and conserved in 4% formaldehyde, treatment which provides proper characteristics to the patch including resistance, flexibility, and lack of antigenicity. The bovine pericardium patch is sewn on the lateral edges to form a 2 cm × 2 cm pouch structure (Figure 3). Following thoracotomy, the exposed descending thoracic aorta is clamped and a 2 cm aortotomy is created. Then, the pericardium pouch is sewn onto the aorta [126] (Figure 3).
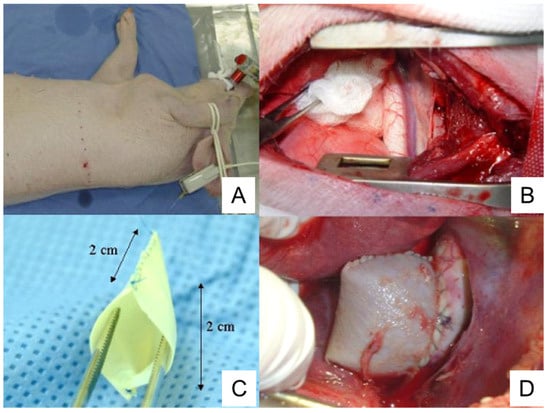
Figure 3. Preparation of porcine TAA using a pericardium pouch. (A), Pig positioning. The animal is laid on their right lateral side. (B), Exposure of thoracic aorta following thoracotomy. (C), Bovine pericardium pouch. (D), Aneurysm sac after the clamps are released. TAA, thoracic aortic aneurysm. The image is from [126], which was published under the terms of the Creative Commons CC BY 4.0 DEED (https://creativecommons.org/licenses/by/4.0/, accessed on 1 November 2023).
Complete endothelization of the aneurysm sac is observed in 50% of animals. Mural thrombi are observed in 80% of animals [126]. An intense healing reaction with myofibroblasts occurs in the periadventitial region, which is not observed in human TAAs [126].
One advantage of the pericardium pouch method is that it can be used to train surgeons and develop new endovascular devices [126], as pigs exhibit anatomic and histopathological characteristics similar to human TAAs. TAAs formed by this method have some characteristics similar to human TAAs, such as the presence of mural thrombi and increased inflammation.
4.5. Cover-Then-Cut Method
A piece of bovine pericardium, the same as the one described in Section 4.4, is tailored into a ~3.5 cm × 4.5 cm oval patch. Following thoracotomy, the porcine ascending aorta is isolated and the pre-prepared patch is sutured to the anterior and bilateral walls (Figure 4A). A longitudinal incision, ~3 cm in length, is made in the anterior wall of the patch. The aortic wall is side-clamped and a hole is made on the aortic wall by cutting away some tissue underneath the patch which connects the pericardial patch cavity with the aortic lumen (Figure 4B). Finally, the patch opening is sutured (Figure 4C) [127].
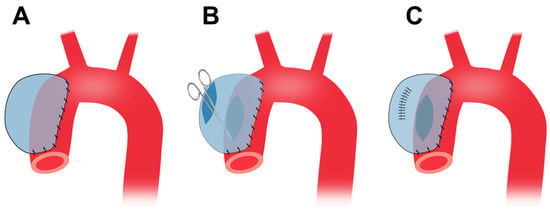
Figure 4. Preparation of porcine TAA using the cover-then-cut method. (A), The commercially available bovine pericardium patch is sewn onto the ascending aortic walls. (B), Following the side clamping, a large hole is made in the aortic wall which connects the patch cavity with the aortic lumen. (C), The patch opening is sutured. TAA, thoracic aortic aneurysm.
This method does not involve cross-clamp of the aorta and the animals do not suffer cross-clamp-associated damages resulting from a temporary stop of blood supply. Therefore, the method provides for limited interference with the circulation system. This method forms TAAs at the ascending aorta originally [127] and can also apply to the descending thoracic region. The wall of the patch aneurysm is smooth and covered by collagen fibers and endothelium six months after the surgery. This model shows gradual TAA growth, and the TAA diameter increases from 48.9 mm at 3 months to 50.3 at 6 months [127].
This model, together with the pericardium pouch method described in Section 4.4, has a number of limitations. First, there are no elastic fibers and smooth muscle cells on the pericardial patch. Second, the TAA formed are saccular whereas in humans TAA is generally fusiform. Third, the etiology is different from human TAAs.
4.6. Media and Intima Resection
This procedure involves cross-clamping of the aorta. To minimize the impact of cross-clamping, all preparation work is conducted before clamping. This includes left thoracotomy, incising the outer layer of the thoracic aorta, detaching the space under the incised membrane, and suturing both edges of the incised adventitia. After these steps, the thoracic aorta is cross-clamped and the aortic media and intima are resected into a spindle shape of about 10 mm to create a tear (Figure 5A). Next, only the adventitial layer is closed using the sutures that had been placed earlier (Figure 5B).
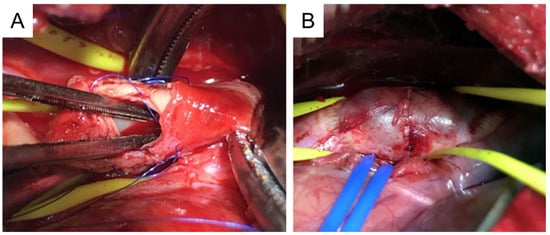
Figure 5. Preparation of porcine TAA using media and intima resection. (A), A primary tear is created at the thoracic aorta. (B), Aneurysm formation at the thoracic aorta. TAA, thoracic aortic aneurysm. This image is from [121] which was published under the terms of the Creative Commons Attribution Non-Commercial No Derivatives (by-nc-nd) License (http://creativecommons.org/licenses/by-nc-nd/4.0/, accessed on 1 November 2023).
The mean time for the whole procedure is ~180 min. The mean aortic clamping time is ~10 min. There is no serious complication associated with the procedure [121]. A saccular TAA is formed in all animals with a mean increase in aortic diameter of 132%.
No patch materials are used, and therefore, the TAAs formed via this method more closely resemble human TAAs compared with the aneurysms created by the previously discussed patch methods. However, this model has a number of limitations. First, it creates a saccular TAA, which is different from a fusiform aortic aneurysm in humans. Second, the TAA formed does not involve atherosclerosis, infiltration of inflammatory cells, and calcification of the aneurysm wall, which are common manifestations of human TAAs.
5. Zebrafish TAA Models
Zebrafish have been widely used as a model organism in biological research [128]. Zebrafish contain gene orthologues relating to 70% of total human genes and 82% of morbidity-associated human genes [129], supporting the relevance of this model for human diseases. Zebrafish models have several advantages: zebrafish (a) are low-cost; (b) have transparent embryos that develop ex utero and therefore permit direct microscopic assessment; (c) can be easily genetically modified, with thousands of transgenic mutated strains or fluorescent reporter lines available; (d) are small in size and require less infrastructure and nursing than mammalian models [128]; and (e) are highly suitable for large scale testing and could be used for unbiased small-molecule suppressor screening, as by day 5 post fertilisation, the aneurysm phenotype has 100% penetrance and animals at this stage are small enough to allow screening in a microwell format [130].
Zebrafish mutant models are used to assess candidate genes associated with TAA [131]. It has been reported that zebrafish develop TAAs at the outflow tract (equivalent to human aortic root) when they are deficient in latent TGFβ-binding protein 1 and 3 [130] and TGFβ receptor 1 [132]. In addition, wild-type zebrafish could develop TAA when they are treated with TGFβ receptor 1 inhibitor LY364947 [130]. Moreover, zebrafish develop aortic aneurysms in the abdominal region when they are treated with angiotensin II or smoke snuff [133].
A limitation of using the zebrafish TAA model is that TAA is developing very fast, i.e., within within 2–5 days post-fertilisation [130,132]. Thus, zebrafish TAAs may not closely mimic human TAAs which develop over decades.
6. Summary of the Animal Models of TAA
The advantages and disadvantages of animal models of TAA are summarized in Table 1.
Table 1. Summary of animal models of TAA.
| Species | Models | Features/Advantages | Disadvantages |
|---|---|---|---|
| Mouse/rat | Overall | Low-cost compared with porcine models. Many GMO animals are available | Not suitable for angiographic imaging and testing TAA repair procedures/devices |
| BAPN | Showing thrombosis, elastic fiber degradation, VSMC apoptosis, and dissection | May not closely represent human TAA development which requires years/decades | |
| AngII | A model of thoracoabdominal aortic aneurysms with dissection | As above | |
| Elastase | TAA location can be controlled | TAAs decrease in size when they reach maximal dilatation at 2 weeks post-surgery | |
| CaCl2 | Showing aortic calcification, apoptosis, inflammation, and ECM degradation | This model is not suitable for investigating TAA progression and rupture | |
| BAPN + AngII | Showing sex phenotype, aortic dissection, intramural hematomas, elastic fiber degradation, and inflammation | May not closely represent human TAA development which requires years/decades | |
| HFD + AngII | Suitable to study TAA-associated dissection | As above | |
| TAC | It mimics pressure overload | The increase in aortic diameter is small | |
| Genetic | Fbn1C1041G/+ and Fbn1mgR/mgR mice are useful for studying Marfan syndrome | Genetic knockout may be lethal | |
| Porcine | Overall | Suitable for angiographic imaging, assessing new devices or interventions | Expensive; Lacking reliable methods to assess anesthetic depth during surgery; GMO pigs are not readily available |
| Elastase | Showing loss of VSMCs and degradation of elastic fibers | As above | |
| Collagenase + CaCl2 | Showing an increase in MMPs, and a decrease in VSMCs | Not showing rupture | |
| Vein patch | Valuable in developing novel treatments for endoleaks after TEVAR | TAAs are histologically different from real aneurysms; TAAs are saccular | |
| Pericardium pouch | Showing mural thrombi and increased inflammation | No elastic fibers and VSMCs on the patch; TAAs formed are saccular; etiology is different from human TAAs | |
| Cover-then-cut | Showing gradual TAA growth | As above | |
| MI resection | TAAs more resemble human TAAs than those derived from patch methods | TAAs formed are saccular; they do not involve inflammatory cell infiltration and calcification | |
| Zebrafish | Genetic and pharmacological | Low-cost; less infrastructure requirement; suitable for direct microscopic assessment and large-scale small-molecule suppressor screening in microwells | TAAs develop within 2–5 days and may not closely mimic human TAAs |
AngII, angiotensin II; BAPN, β-aminopropionitrile; CaCl2, calcium chloride; ECM, extracellular matrix; Fbn1, fibrillin-1; GMO, genetically modified organism; HFD, high-fat diet; MI, media and intima; MMP, matrix metalloproteinase; TAA, thoracic aortic aneurysm; TAC, transverse aortic constriction; TEVAR, thoracic endovascular aortic repair; VSMCs, vascular smooth muscle cells.
This entry is adapted from the peer-reviewed paper 10.3390/ijms25020901
This entry is offline, you can click here to edit this entry!
