Metastasis-associated lung adenocarcinoma transcript-1 (MALAT-1) is a long intergenic non-coding RNA (lncRNA) located on chr11q13. It is overexpressed in several cancers and controls gene expression through chromatin modification, transcriptional regulation, and post-transcriptional regulation. Importantly, MALAT-1 stimulates cell proliferation, migration, and metastasis and serves a vital role in driving the epithelial-to-mesenchymal transition (EMT), subsequently acquiring cancer stem cell-like properties and developing drug resistance. MALAT-1 modulates EMT by interacting with various intracellular signaling pathways, notably the phosphoinositide 3-kinase (PI3K)/Akt and Wnt/β-catenin pathways. It also behaves like a sponge for microRNAs, preventing their interaction with target genes and promoting EMT.
1. Introduction
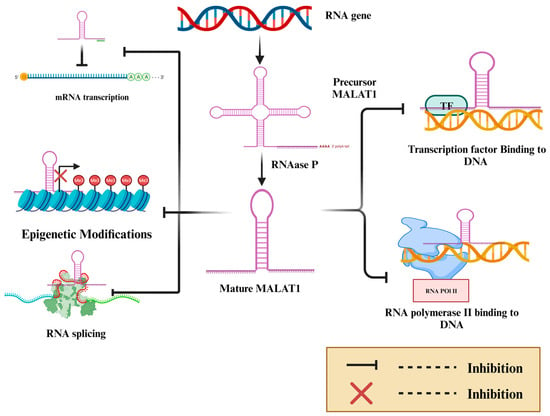
Metastasis-associated lung adenocarcinoma transcript-1 (MALAT-1) is a long non-coding intergenic RNA of 12,820 bases on chr11q13. It is initially transcribed as a precursor transcript, followed by enzymatic processing by RNase P to form the mature long non-coding RNA [
1,
2]. A triple helical structure at the 3′ end stabilizes the structure of MALAT-1 and compensates for the missing poly-A tail. It regulates gene expression by various mechanisms, including modulating gene transcription through the repression of promoters of target genes, regulating RNA-binding proteins or activating mesenchymal transcription factors, modifying the chromatin, and regulating post-transcriptional processing (
Figure 1) [
3,
4,
5,
6]. In addition, it is involved in DNA repair and cell death [
7]. Targeting MALAT-1 induces DNA damage and sensitizes cancer to chemotherapy treatment [
8].
Figure 1. MALAT-1 is an RNA gene encoded by chromosome 11q13. MALAT-1 is initially transcribed as a precursor immature transcript, which the enzyme RNAase P processes to produce mature MALAT-1. MALAT-1 decreases gene expression in many ways, including interfering with a transcription factor, interfering with RNA Pol II, therefore inhibiting transcription, interfering with mRNA splicing, interfering with epigenetic regulation leading to gene silencing and competing with miRNAs, and preventing mRNA transcriptions.
Figure 1 created with
BioRender.com (Accessed on 27 December 2023).
MALAT-1 induces cancer proliferation, invasion, migration, and metastasis to distant sites. Recently, several studies highlighted the immunomodulatory role of MALAT-1 and how it can enable cancer cells to escape immune surveillance by exerting an immunosuppressive effect and regulating the expression of several molecules associated with the tumor microenvironment [
9,
10]. In the triple-negative breast cancer (TNBC) cell model, MALAT-1 knockdown results in a marked induction in MHC class I chain-related proteins A/B expression and the repression of the checkpoint molecules PD-L1 and B7-H4 [
11]. In addition, MALAT1 also modulates its suppressive effect by negatively modulating Myeloid-derived suppressor cells (MDSCs) and decreasing peripheral blood mononuclear cells (PBMCs) in cancer patients [
12]. Indeed, MALAT-1 knockdown using MALAT-1 antisense oligonucleotides (ASO) in an immune-competent mouse model results in a decrease in MDSC as well as immunosuppressive tumor-associated macrophages (TAM). In contrast, an increase in cytotoxic CD8
+ T cells was also observed, which opened new avenues in understanding the conspicuous role of MALAT-1 in modulating carcinogenesis [
13]. Various studies have reported MALAT-1 overexpression in numerous cancers such as esophageal squamous cell carcinoma (ESCC), gastric cancer (GC), non-small cell lung cancer (NSCLC), colorectal cancer (CRC), pancreatic cancer, breast cancer (BC), and hepatocellular carcinoma (HCC) [
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24]. Using the online portal of the University of Alabama at Birmingham (UALCAN) (
https://ualcan.path.uab.edu/) (Accessed on 26 December 2023), researchers analyzed MALAT-1 gene expression across different cancers using data from The Cancer Genome Atlas (TCGA) [
25,
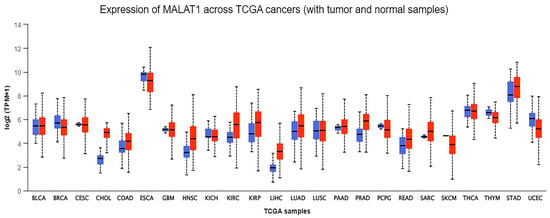
26]. The expression levels of MALAT-1 between samples of different cancers and normal patients show that high expression of MALAT-1 is a prevalent event in various tumors such as ESCC, HCC, cholangiocarcinoma, cervical cancer (CC), sarcoma, and melanoma (
Figure 2). Moreover, plausible differences in the expression of MALAT-1 between normal and cancerous samples were observed in other cancers, such as bladder carcinoma, thyroid carcinoma, and stomach adenocarcinoma (
Figure 2). Contradictory to the disparity in MALAT-1 expression between normal and cancerous samples that are generally observed, samples having approximately the same MALAT-1 expression levels also exist. This ambiguity implies that MALAT-1 has a pleiotropic effect in cancer cells.
Figure 2. Depicts the comparison of MALAT-1 gene expression. MALAT-1 gene expression between tumor samples (denoted in red color) and non-cancerous samples (blue color) across different cancers through the UALCAN database online portal (
https://ualcan.path.uab.edu/) (Accessed on 26 December 2023). The tumor sample shows high expression in various tumors such as esophageal carcinoma, CC, HCC, sarcoma, and melanoma compared to normal patients’ samples.
MALAT-1 is a promising diagnostic marker for detecting endometrial, breast, NSCLC, bladder, and nasopharyngeal carcinoma (NPC) [
27,
28,
29,
30,
31]. However, there is a discrepancy in reporting the diagnostic accuracy of MALAT-1 [
29,
30,
31]. A pooled analysis including 17 studies with 3255 subjects demonstrated that MALAT-1 exhibits moderate accuracy in detecting and diagnosing cancer, and it was strongly associated with the metastasis of early-stage NSCLC [
32]. A wealth of evidence has revealed that the role of MALAT-1 is pivotal in modulating EMT, driving cells to become cancer stem cells (CSCs) or acquire stem cell–like properties, develop chemoresistance, and metastasize to distant places in the body [
17,
33,
34,
35,
36]. Albeit the role of MALAT-1 in cancer has been extensively studied, molecular mechanisms that regulate MALAT-1 are scarcely reported. Several reports examined the expression level of MALAT-1 and its significance as a diagnostic, prognostic, and, recently, as a novel drug target. However, the underlying mechanisms that induce MALAT-1 to play its role are still largely unknown.
In EMT, epithelial cells display migratory and invasive features and become mesenchymal cells via the downregulation of E-cadherin, desmosomes, and claudin, and the upregulation of mesenchymal markers such as N-cadherin, fibronectin, and vimentin [
37,
38,
39,
40,
41,
42]. EMT increases the invasiveness and plasticity of cancer cells, which results in cancer cell dissemination to distant sites through the basement membrane, thereby inducing metastasis. Tumor-associated stroma upregulates the expression of various growth factors such as PDGF, EGF, HGF, and TGF-β, which in turn induces the activation of a series of transcription factors, including SNAI1, Slug, Twist, Zinc finger E-box binding homeobox1 (ZEB1), Goosecoid, and FOXC2, consequently initiating the EMT process [
43,
44,
45,
46,
47,
48]. Several reports demonstrate that EMT is crucial in stimulating cancer progression and metastasis and acquiring drug-resistant properties by modulating alternative cell signaling pathways [
49,
50,
51,
52,
53]. Essential player proteins like Akt, ERK, MAPK, PI3K, β-catenin, and SMAD are essential in modulating EMT by central cell-signaling pathways [
54]. Furthermore, microRNAs also have a crucial role in the cellular signaling circuitry that controls the EMT process. MALAT-1 serves as a competitive endogenous RNA (ceRNA) for tumor-suppressive microRNA and consequently downregulates their gene expression.
2. MALAT-1 Modulates EMT and Promotes Cancer Metastasis, Stemness, and Chemoresistance
This entry is adapted from the peer-reviewed paper 10.3390/cancers16010234