Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Mars is a focus of New Space Age exploration and colonisation, but there are significant challenges to successful colonisation by humankind. Environmental microbes play a key role in supporting the ecosystems of Earth, especially within the biodegradation and bioremediation sectors.
- bioremediation
- terraforming
- nitrogen fixation
- decomposition
- new space age
- Mars
- perchlorate
1. Introduction
Microbes contribute to the fundamental foundations upon which our global biosphere is built [1][2][3][4]. Throughout history, microbes have played key roles in supporting human existence on planet Earth, from the creation of exotic flavours via fermentation [5] in ancient civilisation, to the modern-day use of genetically modified microbes for the creation of biopharmaceutical drugs [6]. As we enter the New Space Age with future manned missions planned to distant planets (Mars being the focus of not only this research but forthcoming NASA missions in the 2030s), it is important to question the reimagined roles of microbes and examine where they belong within the creation of new civilisations on Mars and beyond. While this research focusses on the challenges of colonising Mars, we acknowledge the numerous global challenges to planetary health on Earth, including climate change, environmental destruction, war, famine, and gross social and economic injustices. This raises important ethical debates about investing Earths’ finite resources into the exploration and the terraforming of distant planets, potentially at the expense of improving our own planetary health first. At the same time, exploration of technologies and ecological solutions (including novel application of microbes) is likely to support many terrestrial systems on Earth as well as future strategies to terraform of Mars, thus benefitting both avenues of development alike. Fortunately, planetary exploration and the terraforming of Mars can help support terrestrial systems in many ways thus benefitting Earth and Martian colonisation initiatives alike.
The colonisation of Mars presents challenges that could drive the advancement of cutting-edge technologies. The need for solutions in areas including life support, habitat construction, resource utilisation, and space travel have the potential to spur technological breakthroughs with applications on Earth. These innovations could positively impact two prominent fields causing issues to Earth’s planetary health: renewable energy and healthcare.
Considering the physical environment of Mars, the three main renewable sources of energy consist of solar power, wind power and geothermal energy. Current research into the development of photovoltaic technologies has shown promise for human exploration of Mars [7]. Photovoltaics is the conversion of light into electricity using semiconducting materials that exhibit the photovoltaic effect. As a result, the continual development and reimagination of photovoltaic technology has allowed for the creation of cheap solar energy on Earth, which is both more accessible and greener than fossil fuel to people on Earth [8]. Meanwhile, despite winds on Mars being less powerful than on Earth, this has forced the development of a low Reynolds number vertical axis wind turbine for Mars [9]. This turbine has the added advantage of being able to function midst Martian dust storms. This technology has the potential to revolutionise sub-Saharan communities plagued by drought and consequent dust storms. While geothermal technologies for Mars are yet to emerge for use terrestrially, the continual exploration of geothermal energy on Mars [10] is sure to be the cause of innovative environmentally friendly and accessible technologies on Earth. The insights gained from Mars missions can be applied to address Earth’s resource challenges, including water scarcity and responsible mining practices [11][12][13]. Research into sustainable housing and power provision on Mars, often utilising solar energy, may lead to advancements in efficient space-based housing and energy production, with broader applications on Earth. These technologies contribute to the development of more efficient and sustainable energy solutions [14].
On a humanistic level, the drive to push the boundaries of humanity through space exploration and science can often inspire a new generation of creative thinkers, determined to make global change. Uniting nations with a common goal (for example mRNA vaccine development during the SARS-CoV-2 pandemic [15]) has the potential to revolutionise planetary health, both on Earth and beyond [16]. As of 2023, NASA has funded 8 new studies based on furthering our understanding of human health in relation to space travel. The titles of some of the studies funded include “A time course of bone microarchitectural and material property changes in male and female mice during simulated unloading and spaceflight.” and “Effects of Acute and Protracted Proton Radiation Exposure on Bone Health.” [17]. Meanwhile, rethinking the challenges associated with easily cured first-world human health issues while in space poses its own set of problems, yet the solutions, such as improved telehealth consultations between astronauts and physicians, can aim to have positive knock-on effects terrestrially in communities lacking necessary healthcare [18].
2. A Brief History of Anthropogenic Uses of Bacteria
Throughout history, the relationship between humans and microbes has been dynamic and multifaceted. From co-evolution in prehistory [19] to the profound impacts of the Agricultural Revolution and the challenges of infectious diseases in ancient civilizations [20][21], humans and microbes have influenced each other’s evolution. The Middle Ages witnessed devastating pandemics, while the Scientific Revolution and the 19th century marked breakthroughs in understanding microbiology and disease transmission. The 20th century brought about advancements including vaccines and antibiotics, altering the course of infectious diseases. In the contemporary era, ongoing research of the human microbiome highlights the intricate interplay between microbes and human health.
2.1. Bacterial Uses in Ancient Civilisation
Evolving alongside bacteria, humans have been using the microbial fermentation process to produce foods for thousands of years [22]. Without micro-organisms the human race would not have had the complex flavours and fragrances created as a result of the chemical by-products of microbial fermentation [23]. This process of strategic enzymatic digestion dates back to at least 3150 B.C. as the Egyptians used Saccharomyces cerevisiae in winemaking. Specifically, Cavalieri (2003) describes taking samples of wine residue found in ancient Egyptian wine jars and subjecting them to molecular sequencing. Through ribosomal DNA extraction, amplification, and sequencing, it was possible to identify the presence of Saccharomyces cerevisiae [22].
2.2. Modern Uses of Bacteria
Food: Currently, fermented foods are increasingly highlighted as an important dietary benefit. These foods include yoghurt, sour cream, sauerkraut, kimchi, and miso, which are produced via modern microbial fermentation techniques using Streptococcus thermophilus, Lactococcus lactis, Leuconostoc mesenteroides, Lactobacillus plantarum, and Tetragenococcus halophilus, respectively [24]. These foods facilitate the creation of reportedly bioactive and nutritive compounds, aiming to help target obesity and inflammation, and to provide a source of beneficial probiotics, as reported by Marco et al. (2017).
While the area of probiotics is seen as a controversial topic amongst researchers due to many unsubstantiated claims, some studies suggest they provide beneficial effects by competing with gastrointestinal pathogens, such as E. coli, for adhesion to the gut lining [25] by excluding and displacing pathogens such as food-borne biofilm-forming pathogens [26] restoring epithelial barrier function [27] and even have the potential for beneficial bacteriocin production [28].
Bacteriocins can be described as ribosomal synthesised peptides that exhibit antimicrobial activities. These peptides can either kill or inhibit closely related strains of bacteria, or non-related bacteria, without harming itself, or any other bacteria in the surrounding microbial community [29]. They are used more commonly in today’s society in food technology as preservatives. Due to their peptide nature and protease sensitivity, once ingested they become inactivated by the body’s digestive processes and are generally regarded as safe [30][31]. In 1928, nisin, produced by Lactococcus lactis, was the first of these food-preserving peptides discovered and has since been commercialised and marketed as Nisaplin® [32]. While useful as a sustainably produced food preservative to New Space Age colonists, nisin variants are under constant construction via genetic modification, thus expanding its range of use to future colonists [33].
Genetic Engineering & Recombinant Biopharmaceuticals: While engineered bioremediation microbes have shown promise in the field of oil spill remediation, removal of heavy metals, pesticide remediation and even plastic waste degradation [34][35][36][37], their direct application in nature has not been pursued due to the ethical risks associated with the release of genetically modified organisms into the environment and their potential for associated horizontal gene transfer. Nonetheless, the therapeutic products associated with contained modified microbes have revolutionised the pharmaceutical industry. The global pharmaceutical market was worth $934.8 billion in 2017, with expectations that it will reach $1.9 trillion by 2027, according to IQVIA global contract research organisation [38]. In 1978, Genentech’s David Goeddel created the world’s first recombinant human insulin, using Escherichia coli K12 as the host system to express both the insulin α-chains and β-chains to create a safe and effective form of human insulin. The creation of Humulin R (rapid acting) and Humulin N (NPH, intermediate acting) ensued and heralded in a new age of medicine, by means of recombinant DNA biopharmaceuticals produced using bacterial vectors [39].
Medicine: A pivotal moment within the 21st century’s medical history was the discovery of antibiotics. The year of 1928 brought with it a worldwide revolution as Sir Alexander Fleming first discovered penicillin [40]. Over the coming decades, novel and effective antibiotics were being discovered from soil samples worldwide and this era (between 1950–1970) is now referred to as “The Golden Age of Antibiotic Discovery”.
In today’s modern world, the human race has entered the termed “Age of Disenchantment” as anti-microbial resistance (AMR) is increasing [41]. According to the Centre for Disease Control (CDC) in the U.S.A, at least 2 million people, each year, become infected with antibiotic resistant bacteria, and of those 2 million, at least 23,000 people die. It is speculated that by 2050 antibiotic resistant ‘superbugs’ will be responsible for the deaths of over 10 million people each year, surpassing the death toll caused by cancer [41]. While modern antibiotic discovery may be slowing down, the use of recombinant pharmaceuticals serves as a promising and sustainable option for New Space Age societies.
Throughout human history microbes have played a significant role in supporting humanity by providing key life-building services such as food and medicine production, yet the question remains regarding their potential role in the colonisation of Mars.
3. The Role of Bacteria in Combatting New Space Age Challenges
The Mars Exploration Programme consisting of previous missions including The Mars Reconnaissance Orbiter, The Mars Exploration Rovers, The Curiosity Rover, The Phoenix Lander, and in more recent times, The Opportunity Rover, have all paved the way in increasing knowledge of the Martian landscape. Recent and future missions to Mars include NASA’s 2020 Rover, which aims to study Martian astrobiology, the Mangalyaan Orbiter 2 in 2022, and the Martian Moons Exploration in 2024, which aims to collect samples from the orbiting moons Phobos and Deimos. There are also plans to have manned missions to Mars as early as the 2030s. Key areas that pose a challenge to the colonisation of Mars include:
(A) The Physical Environment.
(B) The Creation of a Hospitable Environment Via Terraforming.
(C) Environmental Sustainability & Life Support.
(D) Environmentally Sustainable Renewable Processing Technologies.
The use of bacteria in facing these Martian colonial challenges (see Figure 1) will be crucial in achieving these goals.
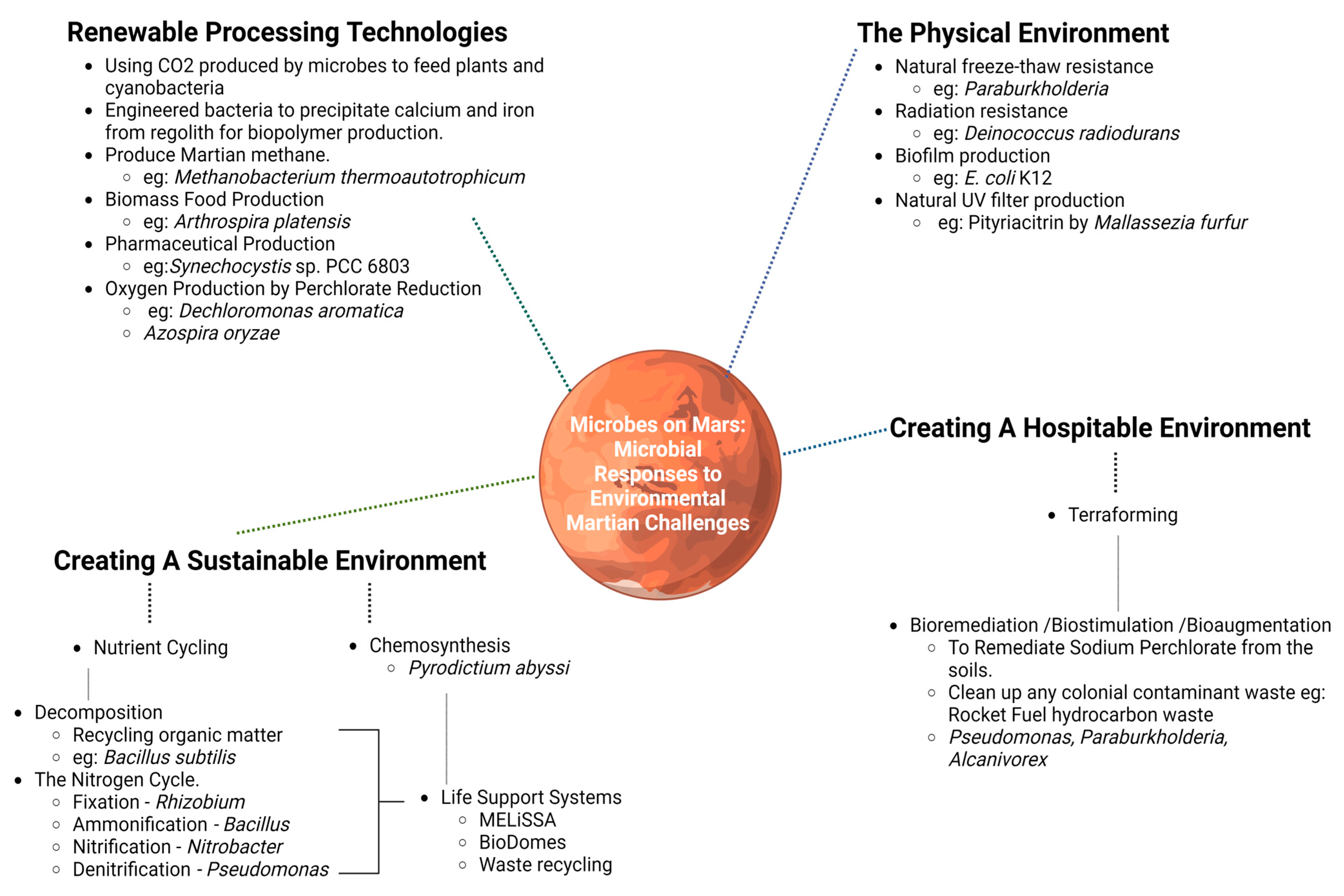
Figure 1. The transferable uses of bacteria on Mars, with examples of associated bacteria and their abilities.
3.1. The Physical Environment
Mars, characterised by its arid and desolate landscape, holds considerable potential for future human colonisation. A significant portion of Mars’ surface is covered in a thick layer of dust primarily composed of iron (III) oxide [42]. The Martian soil, constituting the fine regolith and dust that makes up the surface, is toxic due to elevated concentrations of perchlorate compounds (0.5–1%) [43][44]. Perchlorate, typically found as the anion component of salts associated with cations including ammonium, sodium, or potassium, is considered both a potent oxidizing anion and a health hazard to humans [45]. The future application of bioremediation bacteria holds promise for detoxifying Martian soils, enabling crop cultivation, and facilitating successful human colonization.
The surface temperatures on Mars, fluctuating over its approximately 24.65-h day, vary based on location and reporting sources [46][47]. At the equator, noon temperatures can reach 30 °C, while at the poles, they can plummet to −140 °C, averaging at −63 °C [48]. Mars’ present-day UV wavelengths (200 nm–400 nm) closely resemble those reaching Earth’s surface (290 nm–400 nm) [49]. Notably, Mars’ UV radiation environment features shorter wavelengths in the UVC (200 nm–280 nm) and UVB (280 nm–315 nm) spectra, with UVC posing biological risks due to its DNA-disrupting capabilities [50]. UVC energy absorption by DNA leads to the dimerization of nucleic acid bases, including cyclobutane pyrimidine species and pyrimidine (6–4) pyrimidone compounds, along with their Dewar isomers [51].
One study that highlighted the effects of both freeze-thaw and UVC radiation individually and in tandem on bioremediation strains, demonstrated that some bacteria can withstand up to 20 freeze-thaw cycles in the absence of a cryoprotectant, along with large UVC doses, while the combination of these environmental assaults can either make these strains more UV resistant or sensitive, species dependent [52].
One way to protect these valuable bacteria from thermal fluctuations and exposure is to introduce bioreactors capable of withstanding extreme temperatures, while keeping the enclosed bacteria at a constant temperature. The ISA has recently employed a photobioreactor housing a species of algae called Chlorella vulgaris, which is responsible for both oxygen production and for providing a source of food [53].
To protect against solar UV exposure, the implementation of a physical UV filter that blocks harmful wavelengths could be employed, or a biological filter. One biological filter is pityriacitrin; a potent UV filter produced by Mallassezia furfur, hypothesised to help reduce fungal UV sensitivity and thereby act as an advantage while residing commensally on the skin [54].
Biofilms are an extra-cellular polymeric matrix secreted by bacteria. These allow for adherence to a surface, provide protection against external forces and facilitate effective proliferation [55] thereby serving as a protection to the physical environment. Experimental evidence gathered from the EXPOSE-R2 facilities onboard the ISS detail how both hot and cold desert strains of Chroococcidiopsis survive when exposed to space vacuum, Martian atmosphere, UVC radiation and temperature extremes, due to an ability to form biofilms [56]. Communal biofilms are those which consist of two or more species of bacteria, and they behave symbiotically. Certain strains of E. coli can produce strong biofilms, which could be used in a similar way as Chroococcidiopsis, in order to produce a shielding effect to other bacterial species from stimuli such as UVC rays, when grown in a communal biofilm [57][58]. One promising bacterium for communal-extraterrestrial biofilms is Deinococcus radiodurans, a non-pathogenic coccus, well known for its ability to withstand more than 1500 kilorads of gamma radiation without dying or mutating [59]. Owing to the fact it carries multiple copies of its genome, D. radiodurans can repair any DNA strand breakages caused by ionising radiation and thus might be expected to ensure the long-term survival of a communal biofilm. It is also highly freeze-thaw resistant [52].
In addition to using technological and naturally occurring mechanisms of protecting bacteria against the harsh Martian climate, genetic engineering opens a new frontier of possibilities and brings with it a consideration of bioforming, or of genetically modifying all inhabitants [60]. However, while it is a valid option, the power of genetics is not fully realised and large-scale manipulations to bacterial genomes, or the creation of a perfectly suited synthetic microbe, may prove to be more dangerous to human-life than helpful.
What was originally reported to be a modern adaptation in response to the selective pressures of antibiotic overuse, has since been proven to be a biological and natural trait found in bacteria as a recent metagenomic analysis of 30,000-year-old Beringian permafrost bacterial DNA details the discovery of genes encoding for resistance to β-lactam, tetracycline, and glycopeptide antibiotics. Many antimicrobials are found in nature–for example β-lactam antibiotics are a broad group of molecules that are naturally produced by different organisms (moulds belonging to Penicillium spp. and Cephalosporium spp. for penicillins and cephalosporins, respectively), and bacteria belonging to different species for monobactams and carbapenems [61], so it might be expected that antimicrobial resistance might precede the antibiotic era.
Interestingly, antimicrobial resistance stems beyond the realms of Earth and has also been found in outer space, as bacteria subjected to experimentation on the International Space Station (ISS) have yielded potentially concerning results. Comparisons of biofilm-formation, conjugative transfer and antibiotic resistance was carried out in Staphylococcus and Enterococcus isolates from the ISS, and it was found that biofilm formation was observed in 83% of the isolates; they also possessed multiple genes encoding the resistance of chloramphenicol and erythromycin, along with a higher gene transfer capacity than their terrestrial counter-parts due to the presence of three vir signature genes; virB1, virB4 and virD4 [62]. It is speculated that this is a function of how bacteria react at both a physical and biomolecular level under zero-gravity conditions, along with the absence of a process known as fluid shear. Within the human body, bacteria encounter many different fluids, respective of their location, such as blood, mucous or stomach fluid. These fluids exert a mechanical force on the bacteria, causing shear of their cell walls. It has been postulated that this shear within a bacterium’s surroundings dictates how it behaves [63][64]. It was noted how areas within the body including the intestinal tract, the respiratory area, and the urogenital area, are all areas with low fluid shear, but are also very common sites of infection [65]. Coincidentally, areas of microgravity, such as those found extra-terrestrially, happen to be low-shear environments.
3.2. Terraforming
Terraforming, translated as Earth-shaping, can be described as the deliberate modification of a planet, moon or celestial body’s atmosphere, biosphere (temperature and ecology), and surface topography, to create a habitable environment similar to that of Earth. The terraforming of Mars is a multi-faceted operation, which, according to Haynes and McKay (1992), involves three main steps, namely the human/robotic exploration of Mars (which has been achieved through various Mars landers); the planetary engineering step, designed to warm the planet, liberate liquid water and produce a thick carbon dioxide atmosphere; and the introduction of pioneering microbial communities able to proliferate in the Martian environment [66]. While there are numerous, and technologically feasible, answers as to how humankind would conduct the planetary engineering steps, such as using orbital mirrors, redirecting ammonia asteroids and producing halocarbons [67], the introduction of suitable bioremedial microbial communities is an emerging and important area of research.
3.3. Environmental Sustainability via Microbial Action
One initiative in ensuring human survival during space travel and the establishment of human and microbial colonies on the Martian landscape is the use of self-sustained and controlled environments, built upon the foundation of nutrient cycling.
3.4. Renewable Processing Technologies
While looking towards the future uses of microbes on Mars resources are finite and colonisation requires renewable microbial-based technologies. If mankind colonises Mars, these colonists must utilise all available resources. As a result, biological processing technology must be developed for different kinds of resources found within the environment; mainly for solid waste (produced by the actions of those onboard spacecrafts), volatiles (mainly occurring with the life support systems) and minerals/geological materials (from the planet’s surface itself, and asteroids). The anaerobic process of breaking down human waste by bacteria has revealed the production of nitrous oxide, a fuel for space travel [68]. Volatiles such as carbon dioxide can be utilised by either higher plants or cyanobacteria, as discussed earlier, which can be found in the life support systems. The use of engineered bacteria to precipitate calcium/iron from regolith and other geological materials, in order to make bio-cements and construct bio-polymers for in situ manufacturing is another possible use of resources by means of bacterial action [69]. This in turn will allow for the steady colonisation of Mars as buildings can be constructed and the landscape will be altered to fit human needs in the new space age.
In the only publication to date on the subject it has been calculated that during a 916-day mission to Mars, the use of Arthrospira maxima and Arthrospira platensis over a 496-day period could produce enough biomass on Mars to decrease the shipped wet-food mixed-menu on a one-way-journey by 38%; using Cupriavidus necator over 202 days for Martian polyhydroxybutyrate synthesis can lower the total mass shipped to Mars by 85%, and instead allow for the production of a 120 m3 six-person habitat by means of 3-D printing; the use of Methanobacterium thermoautotrophicum could reduce manufacturing mass of Martian fuel (methane) by 56% based on 205 days of Martian bioproduction. Lastly, as an example, a few days of acetaminophen production by Synechocystis sp. PCC 6803 could restock used pharmaceuticals or those which have been exposed to irradiation or have become expired, thus eliminating the pressures faced by the conservation of certain medical supplies while waiting for future shipments [70].
The Martian landscape, in terms of its suitability for crop cultivation, lacks nitrogen which is necessary for crop growth. Despite a recent discovery by The Curiosity Rover at Gale crater, Mars, which detailed the discovery of sedimentary nitrogen deposits most likely caused by ancient volcanic plume lightning fixation [71], the main source of available nitrogen on Mars is found atmospherically at 2.7% [72]. The use of bacteria which are encoded to over-express the nif genes for nitrogen fixation could help significantly in Martian crop cultivation.
New space age travel to extra-terrestrial colonial sites requires rockets, and rockets require fuel. Another by-product of space shuttle soli-propellants is sodium perchlorate [45][73][74]. Sodium perchlorate is an inorganic, water-soluble compound which has also been found on Mars, as detailed by previous rovers, from inferred brine seeps–a remnant of Mars’ prehistoric past where rivers once carved the topography of the Red Planet [43][75]. Perchlorate poses a significant risk to the health of future colonists as not only is it toxic but can be passed through the food chain via bioaccumulation. This would pose a threat of perchlorate poisoning to colonists growing crops within perchlorate-contaminated soils [76]. One way to combat this threat is the employment of either wildtype perchlorate-reducing bioremediation microbes, such as Dechloromonas aromatica or Azospira oryzae, or even the use of a genetically modified organism, which has been engineered to withstand both the harsh conditions of Mars and is also able to reduce perchlorate. Both of these methods feed into the terraforming stages of Mars. At present, analytical methods for perchlorate sampling and detection within a system are confined to cumbersome and large pieces of equipment such as ion chromatography [77][78]. Recently, a more compact and novel method for sampling and detecting perchlorate within a system has been identified by means of Raman Spectroscopy [79]. Combined with the use of perchlorate-reducing strains, Raman spectral analysis could allow colonists to monitor remediated soils to ensure the complete absence of perchlorate, thus reducing the risk of perchlorate bioaccumulation within crops grown in Martian soils.
Nonetheless, these potential initiatives mentioned to protect bacteria, mainly biological, technological, and even bio-technological, showcase a variety of options that might assist survival, increasing the possibility of one day leaving the Earth for Mars.
This entry is adapted from the peer-reviewed paper 10.3390/challe15010005
References
- Trevors, J.T. Review: From Chemosphere to Biosphere. World J. Microbiol. Biotechnol. 2001, 17, 651–655.
- Vallet, M.; Kaftan, F.; Grabe, V.; Ghaderiardakani, F.; Fenizia, S.; Svatoš, A.; Pohnert, G.; Wichard, T. A New Glance at the Chemosphere of Macroalgal-Bacterial Interactions: In Situ Profiling of Metabolites in Symbiosis by Mass Spectrometry. Beilstein J. Org. Chem. 2021, 17, 1313–1322.
- Oliveira, V.M.; Andreote, F.D.; Cortelo, P.C.; Castro-Gamboa, I.; Costa-Lotufo, L.V.; Polizeli, M.d.L.T.M.; Thiemann, O.H.; Setubal, J.C. Microorganisms: The Secret Agents of the Biosphere, and Their Key Roles in Biotechnology. Biota Neotrop. 2022, 22, e20221343.
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ Warning to Humanity: Microorganisms and Climate Change. Nat. Rev. Microbiol. 2019, 17, 569–586.
- Mannaa, M.; Han, G.; Seo, Y.-S.; Park, I. Evolution of Food Fermentation Processes and the Use of Multi-Omics in Deciphering the Roles of the Microbiota. Foods 2021, 10, 2861.
- Hutchinson, C.R. Drug Synthesis by Genetically Engineered Microorganisms. Biotechnology 1994, 12, 375–380.
- Abel, A.J.; Berliner, A.J.; Mirkovic, M.; Collins, W.D.; Arkin, A.P.; Clark, D.S. Photovoltaics-Driven Power Production Can Support Human Exploration on Mars. Front. Astron. Space Sci. 2022, 9, 868519.
- Breyer, C. Low-Cost Solar Power Enables a Sustainable Energy Industry System. Proc. Natl. Acad. Sci. USA 2021, 118, e2116940118.
- Kumar, V.; Paraschivoiu, M.; Paraschivoiu, I. Low Reynolds Number Vertical Axis Wind Turbine for Mars. Wind. Eng. 2010, 34, 461–476.
- Morgan, P. Geothermal Energy on Mars. In Mars; Springer: Berlin/Heidelberg, Germany, 2009; pp. 331–349. ISBN 978-3-642-03629-3.
- Santomartino, R.; Zea, L.; Cockell, C.S. The Smallest Space Miners: Principles of Space Biomining. Extremophiles 2022, 26, 7.
- Vincendon, M.; Mustard, J.; Forget, F.; Kreslavsky, M.; Spiga, A.; Murchie, S.; Bibring, J. Near-tropical Subsurface Ice on Mars. Geophys. Res. Lett. 2010, 37, L01202.
- Mellerowicz, B.; Zacny, K.; Palmowski, J.; Bradley, B.; Stolov, L.; Vogel, B.; Ware, L.; Yen, B.; Sabahi, D.; Ridilla, A.; et al. RedWater: Water Mining System for Mars. New Space 2022, 10, 166–186.
- Soureshjani, O.K.; Massumi, A.; Nouri, G. Sustainable Colonization of Mars Using Shape Optimized Structures and in Situ Concrete. Sci. Rep. 2023, 13, 15747.
- Matarazzo, L.; Bettencourt, P.J.G. MRNA Vaccines: A New Opportunity for Malaria, Tuberculosis and HIV. Front. Immunol. 2023, 14, 1172691.
- Lawler, A. Collaborative Research. Plans for Mars Unite Cancer, Space Agencies. Science 2000, 288, 415–416.
- Dunbar, B.; Graf, A. NASA Funds Eight Studies to Protect Astronaut Health on Long Missions-NASA. Available online: https://www.nasa.gov/humans-in-space/nasa-funds-eight-studies-to-protect-astronaut-health-on-long-missions/ (accessed on 6 December 2023).
- Lovett, L. Mission to Mars: The Healthcare Challenges Facing NASA|MobiHealthNews. 2019. Available online: https://www.mobihealthnews.com/news/mission-mars-healthcare-challenges-facing-nasa (accessed on 17 September 2023).
- Shahab, M.; Shahab, N. Coevolution of the Human Host and Gut Microbiome: Metagenomics of Microbiota. Cureus 2022, 14, e26310.
- Harper, K.N.; Armelagos, G.J. Genomics, the Origins of Agriculture, and Our Changing Microbe-Scape: Time to Revisit Some Old Tales and Tell Some New Ones. Am. J. Phys. Anthr. 2013, 152 (Suppl. S57), 135–152.
- Rodríguez-Frías, F.; Quer, J.; Tabernero, D.; Cortese, M.F.; Garcia-Garcia, S.; Rando-Segura, A.; Pumarola, T. Microorganisms as Shapers of Human Civilization, from Pandemics to Even Our Genomes: Villains or Friends? A Historical Approach. Microorganisms 2021, 9, 2518.
- Cavalieri, D.; McGovern, P.E.; Hartl, D.L.; Mortimer, R.; Polsinelli, M. Evidence for S. Cerevisiae Fermentation in Ancient Wine. J. Mol. Evol. 2003, 57, S226–S232.
- Hagedorn, S.; Kaphammer, B. Microbial Biocatalysis in the Generation of Flavor and Fragrance Chemicals. Annu. Rev. Microbiol. 1994, 48, 773–800.
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gä Nzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102.
- Lee, Y.-K.; Puong, K.-Y. Competition for Adhesion between Probiotics and Human Gastrointestinal Pathogens in the Presence of Carbohydrate. Br. J. Nutr. 2002, 88, S101.
- Woo, J.; Ahn, J. Probiotic-Mediated Competition, Exclusion and Displacement in Biofilm Formation by Food-Borne Pathogens. Lett. Appl. Microbiol. 2013, 56, 307–313.
- Ewaschuk, J.B.; Diaz, H.; Meddings, L.; Diederichs, B.; Dmytrash, A.; Backer, J.; Looijer-van Langen, M.; Madsen, K.L. Secreted Bioactive Factors from Bifidobacterium Infantis Enhance Epithelial Cell Barrier Function. Am. J. Physiol.-Gastrointest. Liver Physiol. 2008, 295, G1025–G1034.
- Dobson, A.; Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocin Production: A Probiotic Trait? Appl. Environ. Microbiol. 2012, 78, 1–6.
- Yang, S.-C.; Lin, C.-H.; Sung, C.T.; Fang, J.-Y. Antibacterial Activities of Bacteriocins: Application in Foods and Pharmaceuticals. Front. Microbiol. 2014, 5, 241.
- Bernbom, N.; Licht, T.R.; Brogren, C.-H.; Jelle, B.; Johansen, A.H.; Badiola, I.; Vogensen, F.K.; Norrung, B. Effects of Lactococcus Lactis on Composition of Intestinal Microbiota: Role of Nisin. Appl. Environ. Microbiol. 2006, 72, 239–244.
- Cleveland, J.; Montville, T.J.; Nes, I.F.; Chikindas, M.L. Bacteriocins: Safe, Natural Antimicrobials for Food Preservation. Int. J. Food Microbiol. 2001, 71, 1–20.
- Rogers, L.A. The Inhibiting Effect of Streptococcus Lactis on Lactobacillus Bulgaricus. J. Bacteriol. 1928, 16, 321–325.
- Zheng, Y.; Du, Y.; Qiu, Z.; Liu, Z.; Qiao, J.; Li, Y.; Caiyin, Q. Nisin Variants Generated by Protein Engineering and Their Properties. Bioengineering 2022, 9, 251.
- Goudriaan, M.; Morales, V.H.; van der Meer, M.T.J.; Mets, A.; Ndhlovu, R.T.; van Heerwaarden, J.; Simon, S.; Heuer, V.B.; Hinrichs, K.-U.; Niemann, H. A Stable Isotope Assay with 13C-Labeled Polyethylene to Investigate Plastic Mineralization Mediated by Rhodococcus Ruber. Mar. Pollut. Bull. 2023, 186, 114369.
- Saravanan, A.; Kumar, P.S.; Ramesh, B.; Srinivasan, S. Removal of Toxic Heavy Metals Using Genetically Engineered Microbes: Molecular Tools, Risk Assessment and Management Strategies. Chemosphere 2022, 298, 134341.
- Singh, D.K. Biodegradation and Bioremediation of Pesticide in Soil: Concept, Method and Recent Developments. Indian. J. Microbiol. 2008, 48, 35–40.
- French, K.E.; Zhou, Z.; Terry, N. Horizontal ‘Gene Drives’ Harness Indigenous Bacteria for Bioremediation. Sci. Rep. 2020, 10, 15091.
- IQVIA Institute Global Market for Medicines to Rise to $1.9 Trillion by 2027, Says Report from IQVIA Institute-IQVIA. Available online: https://www.iqvia.com/newsroom/2023/01/global-market-for-medicines-to-rise-to-19-trillion-by-2027-says-report-from-iqvia-institute (accessed on 19 April 2023).
- Quianzon, C.C.; Cheikh, I. History of Insulin. J. Community Hosp. Intern. Med. Perspect. 2012, 2, 18701.
- Fleming, A. Classics in Infectious Diseases: On the Antibacterial Action of Cultures of a Penicillium, with Special Reference to Their Use in the Isolation of B. Influenzae by Alexander Fleming, Reprinted from the British Journal of Experimental Pathology 10:226–236. Rev. Infect. Dis. 1929, 2, 129–139.
- CDC Antibiotic/Antimicrobial Resistance|CDC. Available online: https://www.cdc.gov/drugresistance/index.html (accessed on 17 October 2018).
- Peplow, M. How Mars Got Its Rust. Nature 2004.
- Hecht, M.; Kounaves, S.; Quinn, R.; West, S.; Young, S.; Ming, D.; Catling, D.; Clark, B.; Boynton, W.; Hoffman, J.; et al. Detection of Perchlorate and the Soluble Chemistry of Martian Soil at the Phoenix Lander Site. Science 2009, 325, 64–67.
- Davila, A.F.; Willson, D.; Coates, J.D.; McKay, C.P.; Davila, A.F.; Willson, D.; Coates, J.D.; McKay, C.P. Perchlorate on Mars: A Chemical Hazard and a Resource for Humans. Int. J. Astrobiol. 2013, 12, 321–325.
- Srinivasan, A.; Viraraghavan, T. Perchlorate: Health Effects and Technologies for Its Removal from Water Resources. Int. J. Environ. Res. Public Health 2009, 6, 1418–1442.
- Sinton, W.M. Taking the Temperatures of the Moon and Planets. Astron. Soc. Pac. Leafl. 1958, 7, 361.
- Moissl-Eichinger, C.; Cockell, C.; Rettberg, P. Venturing into New Realms? Microorganisms in Space. FEMS Microbiol. Rev. 2016, 40, 722–737.
- NASA Mars Facts|All about Mars–NASA’s Mars Exploration Program. Available online: https://mars.nasa.gov/all-about-mars/facts/ (accessed on 13 April 2020).
- Catling, D.C.; Cockell, C.S.; Mckay, C.P. Ultraviolet Radiation on the Surface of MARS. In Proceedings of the Fifth International Conference on Mars, Pasadena, CA, USA, 18–23 June 1999.
- Cockell, C.S.; Catling, D.C.; Davis, W.L.; Snook, K.; Kepner, R.L.; Lee, P.; McKay, C.P. The Ultraviolet Environment of Mars: Biological Implications Past, Present, and Future. Icarus 2000, 146, 343–359.
- Pattison, D.I.; Davies, M.J. Actions of Ultraviolet Light on Cellular Structures. In Cancer: Cell Structures, Carcinogens and Genomic Instability; Birkhäuser-Verlag: Basel, Switzerland, 2006; pp. 131–157.
- Keaney, D.; Lucey, B.; Quinn, N.; Finn, K. The Effects of Freeze-Thaw and UVC Radiation on Microbial Survivability in a Selected Mars-like Environment. Microorganisms 2022, 10, 576.
- Mathewson, S. Algae “Bioreactor” on Space Station Could Make Oxygen, Food for Astronauts|Space. Available online: https://www.space.com/space-station-algae-experiment-fresh-air.html (accessed on 11 June 2019).
- Machowinski, A.; Kramer, H.-J.; Hort, W.; Mayser, P. Pityriacitrin ? A Potent UV Filter Produced by Malassezia Furfur and Its Effect on Human Skin Microflora. Mycoses 2006, 49, 388–392.
- Besemer, K. Biodiversity, Community Structure and Function of Biofilms in Stream Ecosystems. Res. Microbiol. 2015, 166, 774–781.
- Baqué, M.; de Vera, J.-P.; Rettberg, P.; Billi, D. The BOSS and BIOMEX Space Experiments on the EXPOSE-R2 Mission: Endurance of the Desert Cyanobacterium Chroococcidiopsis under Simulated Space Vacuum, Martian Atmosphere, UVC Radiation and Temperature Extremes. Acta Astronaut. 2013, 91, 180–186.
- DePas, W.H.; Syed, A.K.; Sifuentes, M.; Lee, J.S.; Warshaw, D.; Saggar, V.; Csankovszki, G.; Boles, B.R.; Chapman, M.R. Biofilm Formation Protects Escherichia Coli against Killing by Caenorhabditis Elegans and Myxococcus Xanthus. Appl. Environ. Microbiol. 2014, 80, 7079–7087.
- Liu, W.; Røder, H.L.; Madsen, J.S.; Bjarnsholt, T.; Sørensen, S.J.; Burmølle, M. Interspecific Bacterial Interactions Are Reflected in Multispecies Biofilm Spatial Organization. Front. Microbiol. 2016, 7, 1366.
- Makarova, K.S.; Aravind, L.; Wolf, Y.I.; Tatusov, R.L.; Minton, K.W.; Koonin, E.V.; Daly, M.J. Genome of the Extremely Radiation-Resistant Bacterium Deinococcus Radiodurans Viewed from the Perspective of Comparative Genomics. Microbiol. Mol. Biol. Rev. 2001, 65, 44–79.
- Sleator, R.D.; Smith, N. Directed Panspermia: A 21st Century Perspective. Sci. Prog. 2017, 100, 187–193.
- De Rosa, M.; Verdino, A.; Soriente, A.; Marabotti, A. The Odd Couple(s): An Overview of Beta-Lactam Antibiotics Bearing More Than One Pharmacophoric Group. Int. J. Mol. Sci. 2021, 22, 617.
- Schiwon, K.; Arends, K.; Rogowski, K.M.; Fürch, S.; Prescha, K.; Sakinc, T.; Van Houdt, R.; Werner, G.; Grohmann, E. Comparison of Antibiotic Resistance, Biofilm Formation and Conjugative Transfer of Staphylococcus and Enterococcus Isolates from International Space Station and Antarctic Research Station Concordia. Microb. Ecol. 2013, 65, 638–651.
- Nickerson, C.A.; Ott, C.M.; Wilson, J.W.; Ramamurthy, R.; Pierson, D.L. Microbial Responses to Microgravity and Other Low-Shear Environments. Microbiol. Mol. Biol. Rev. 2004, 68, 345–361.
- Castro, S.L.; Nelman-Gonzalez, M.; Nickerson, C.A.; Ott, C.M. Induction of Attachment-Independent Biofilm Formation and Repression of Hfq Expression by Low-Fluid-Shear Culture of Staphylococcus Aureus. Appl. Environ. Microbiol. 2011, 77, 6368–6378.
- Love, S. Bacteria Get Dangerously Weird in Space|Indy100. Available online: https://www.indy100.com/article/bacteria-get-dangerously-weird-in-space-7380481 (accessed on 29 October 2018).
- Haynes, R.H.; McKay, C.P. The Implantation of Life on Mars: Feasibility and Motivation. Adv. Space Res. 1992, 12, 133–140.
- Zubrin, R.; McKay, C. Technological Requirements for Terraforming Mars. Available online: http://www.users.globalnet.co.uk/~mfogg/zubrin.htm (accessed on 10 November 2018).
- Scherson, Y.D.; Wells, G.F.; Woo, S.-G.; Lee, J.; Park, J.; Cantwell, B.J.; Criddle, C.S. Nitrogen Removal with Energy Recovery through N2O Decomposition. Energy Environ. Sci. 2013, 6, 241–248.
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial Carbonate Precipitation in Construction Materials: A Review. Ecol. Eng. 2010, 36, 118–136.
- Menezes, A.A.; Cumbers, J.; Hogan, J.A.; Arkin, A.P. Towards Synthetic Biological Approaches to Resource Utilization on Space Missions. J. R. Soc. Interface 2015, 12, 20140715.
- Stern, J.C.; Sutter, B.; Freissinet, C.; Navarro-González, R.; McKay, C.P.; Archer, P.D.; Buch, A.; Brunner, A.E.; Coll, P.; Eigenbrode, J.L.; et al. Evidence for Indigenous Nitrogen in Sedimentary and Aeolian Deposits from the Curiosity Rover Investigations at Gale Crater, Mars. Proc. Natl. Acad. Sci. USA 2015, 112, 4245–4250.
- Williams, D. Mars Fact Sheet. Available online: https://nssdc.gsfc.nasa.gov/planetary/factsheet/marsfact.html (accessed on 23 April 2019).
- Motzer, W. Perchlorate: Problems, Detection, and Solutions. Environ. Forensics 2001, 2, 301–311.
- Renner, R. Food Safety: Perchlorate Exposure: Tip of the Iceberg? Environ. Health Perspect. 2005, 113, A232.
- Glavin, D.P.; Freissinet, C.; Miller, K.E.; Eigenbrode, J.L.; Brunner, A.E.; Buch, A.; Sutter, B.; Archer, P.D.; Atreya, S.K.; Brinckerhoff, W.B.; et al. Evidence for Perchlorates and the Origin of Chlorinated Hydrocarbons Detected by SAM at the Rocknest Aeolian Deposit in Gale Crater. J. Geophys. Res. Planets 2013, 118, 1955–1973.
- Becker, C. Prophylaxis and Treatment of Side Effects Due to Iodinated Contrast Media Relevant to Radiological Practice. Radiologe 2007, 47, 768–773.
- Seiler, M.A.; Jensen, D.; Neist, U.; Deister, U.K.; Schmitz, F. Validation Data for the Determination of Perchlorate in Water Using Ion Chromatography with Suppressed Conductivity Detection. Environ. Sci. Eur. 2016, 28, 18.
- Seiler, M.A.; Jensen, D.; Neist, U.; Deister, U.K.; Schmitz, F. Determination of Trace Perchlorate in Water: A Simplified Method for the Identification of Potential Interferences. Environ. Sci. Eur. 2017, 29, 30.
- Luo, Y.; Naidu, R.; Fang, C. Raman Imaging towards In-Situ Visualisation of Perchlorate Adsorption. Water Res. 2023, 229, 119510.
This entry is offline, you can click here to edit this entry!
