BCFAs, the bacterial products of the catabolism of branched-chain amino acids, are proposed as markers for colonic protein fermentation. IBS is a gastrointestinal disorder characterized by low-grade inflammation and intestinal dysbiosis. The low-FODMAP diet (LFD) has increasingly been applied as first-line therapy for managing IBS symptoms, although it decreases the production of short-chain fatty acids (SCFA), well known for their anti-inflammatory action. In parallel, high protein consumption increases BCFAs. Protein fermentation alters the colonic microbiome through nitrogenous metabolites production, known for their detrimental effects on the intestinal barrier promoting inflammation.
1. Introduction
Irritable bowel syndrome (IBS) is a highly prevalent disorder characterized by abdominal pain and changes in bowel habits. Its etiology is still unknown, although patients often report an infectious or traumatic event triggering the onset of symptoms. The pathogenetic mechanisms include, among others, increased intestinal permeability, intestinal dysbiosis, visceral hypersensitivity, gut–brain axis dysregulation, low-grade intestinal inflammation, and psychological stress [
1]. Additionally, alterations in intestinal fermentation may lead to abnormalities, such as intraluminal excessive gas production and altered motility, which are common in IBS. Intraluminal intestinal fermentation by colonic bacteria produces gases such as hydrogen and carbon dioxide, short-chain fatty acids (SCFAs), and branched-chain fatty acids (BCFAs) as secondary byproducts. SCFAs and BCFAs are natural acids and cause a significant pH drop in the intestinal lumen (the intraluminal pH varies depending on the intestinal segment and ranging from 5.5–7.5 in the cecum/right colon, and to 6.1–7.5 in the left colon and rectum) [
2,
3].
Fatty acids are the most important components of bacterial membrane lipids. BCFAs are a group of saturated lipids presenting in many organisms affecting multiple signaling pathways. BCFAs were first recognized as significant nutrients for the gastrointestinal tract (GI) health in the context of vernix caseosa, the white waxy substance that develops during the last trimester of human fetuses [
4]. BCFAs constitute a considerable component of many gut bacteria (~15% of phyla) [
5]. Although they take part in numerous biochemical procedures, BCFAs are not satisfactorily explored, and research in humans is scarce [
6]. BCFAs, mainly isobutyrate, isovalerate, and 2-methyl butanoate, contribute to as much as 5% of total SCFA production and derive from the metabolism of valine, leucine, and isoleucine, respectively (all belong to essential amino acids) [
7]. BCFAs are highly abundant in the cecum and colon and their levels in fecal samples have inversely correlated to fiber (especially insoluble) consumption [
7,
8]. While the responses to SCFAs in the gut are mediated through the free fatty acid receptors 2 (FFA2) and 3 (FFA3) binding to G-protein (GPR41 and GPR43), little is known about the metabolism of BCFAs in host physiology.
Interconnections between microbes, foods, and hosts in the human gut form a complex ecological network. Gut microbiota produces metabolites through the fermentation of nondigested food components, and specific metabolites are produced depending on the type of food the individual consumes [
12]. Food is one of the factors that trigger symptoms in IBS patients, and specific dietary patterns are the first-line therapeutic approach. The low-FODMAP diet (LFD) is gaining ground as the most well-documented dietary intervention in reducing these symptoms [
13].
FODMAP stands for fermentable oligo-, di-, mono-saccharides, and polyols, a large class of small nondigestible carbohydrates containing up to 10 sugars. Those are poorly absorbed in the small bowel and they are potential triggers for exacerbating abdominal symptoms in IBS patients [
14]. The intolerance to FODMAPs causes luminal distension while other metabolic products, such as pathogen-associated molecules, may induce pain symptoms, particularly in IBS patients with visceral hypersensitivity [
15].
The flow of undigested carbohydrates, especially fibers, into the gut is associated with beneficial effects, as they are preferentially used by many bacterial species as the main energy source to produce SCFAs. SCFAs, especially butyrate, have been related to improved epithelial barrier function and a decrease in pH [
24,
25]. Restricting fermentable substrates for saccharolytic gut bacteria reduces SCFA production [
13]. When saccharolytic fermentation (carbohydrate) is reduced and protein fermentation significantly occurs, elevated concentrations of BCFAs are detected across the colon [
26]. The proteolytic activity in the large intestine has been mainly attributed to the genera
Bacteroides,
Propionibacterium,
Clostridium,
Streptococcus,
Fusobacterium, and
Lactobacillus.
Bacteroides spp. secreting proteases act like elastase and, in the case of their abundance (or overgrowth), they may degrade maltase and sucrase enzymes in the enterocyte brush borders [
27].
Intestinal inflammation status is closely related to gut health, and low-grade inflammation has been detected in the large bowel of patients suffering from IBS. Moreover, consumption of specific food may lead to alteration of the abundance of various microorganisms. The way that food is processed could also lead to inflammation.
BCFAs have not attracted attention like SCFAs, even though they may have a crucial role in the gut milieu and could be considered potential markers of microbial metabolism [
29]. Butyrate is the main energy source of colonocytes, providing 70–80% of their energy requirements and regulating colonic homeostasis, but BCFAs have the potential to be oxidized when butyrate is not available, and isobutyrate could be used as an alternative energy source [
2,
30,
31].
The Role of BCFAs in Intestinal Inflammation
Intestinal inflammation disturbs normal growth in humans and animals and leads to bowel diseases [
33]. Many commensal bacteria utilize BCFAs to survive in the varying milieus. Modulating membrane fluidity is essential for bacterial survival in a variety of environments, and many microorganisms use BCFAs in their membranes to modulate biophysical procedures [
34]. BCFAs are taken up and incorporated into enterocyte membranes where they modulate the inflammatory response [
35,
36,
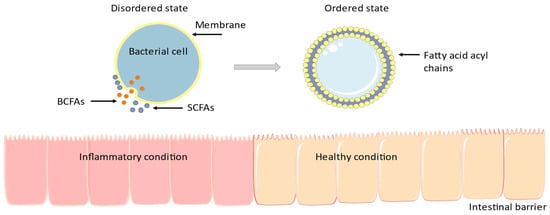
37]. In detail, as shown in
Figure 1, BCFAs affect the cell membrane’s fluidity: with high BCFAs concentrations, membrane falls into a disordered state, allowing better transport, membrane protein structure and functionality, and cellular signal transduction and trafficking.
Figure 1. Many microorganisms use BCFAs in their membranes to modulate biophysical procedures [
34]. BCFAs are taken up and incorporated into enterocyte membranes where they modulate the inflammatory response [
35,
36,
37]. The figure was designed using Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license.
While the three SCFAs (acetate, butyrate, and propionate) produced by fermented nondigestible carbohydrates might inhibit inflammation and exert anti-inflammatory effects via the NF-κB/NLRP3 signaling pathway [
39], the anti-inflammatory potential of dietary BCFAs has been demonstrated in vitro in the human-derived intestinal cell line Caco-2. Exposure of Caco-2 cells to BCFAs decreased the lipopolysaccharide-induced gene expression of proinflammatory mediators (i.e., IL-8, TLR-4, and NF-κB), while shorter-chain BCFAs (branched short-chain carboxylic acids are iso-14:0 and iso-16:0 -series, derived from valine, iso-15:0 and iso-17:0-series from leucine, and anteiso-15:0 and anteiso-17:0-series from isoleucine) incorporated into phospholipids similarly to rat infants’ ileum, and improved the lipopolysaccharide-induced reduction in cell viability [
37,
40].
BCFAs increase protein SUMOylation (small ubiquitin-like modifier, a crucial ubiquitin-like modification involved in numerous intestinal functions) in intestinal cell lines in a pH-dependent manner. Ezzine et al. assessed the role of BCFAs in the inflammatory response and intestinal epithelial integrity in Caco-2 cell cultures. The results revealed that fatty acids produced by gut bacteria could regulate intestinal physiology by modulating SUMOylation and diminishing host inflammatory responses triggered by the gut microbiota.
Necrotizing enterocolitis (NEC) is an inflammatory disease in the GI of premature infants, which is a major cause of morbidity, with an estimated rate of death of 20–30% [
5].
According to Russel [
42] and her colleagues, high-protein and low-carbohydrate diets increase the BCFA levels, the phenylacetic acid concentrations, and N-nitroso compounds. That dietary pattern correlated with reduced abundance of
Roseburia/
Eubacterium rectale, a beneficial bacterium for gut health. Furthermore, high levels of
Faecalibacterium prausnitzii, which is well known for its anti-inflammatory effects on the intestinal mucosa, were detected [
42]. Following a high-protein formula consumption, female piglets displayed decreased levels of
F. prausnitzii and BCFAs [
43].
2. The Low-FODMAP Diet, IBS, and BCFAs
2.1. BCFAs & IBS
Human Studies, Table 1
In a metabolomic study, nuclear magnetic resonance (NMR) spectroscopy was used to identify complex mixture metabolites present in fecal samples to explore differences among IBS (
n = 10), UC (
n = 13), and controls (
n = 22). The results showed lower BCFA production in the IBS group compared to controls [
47]. Some years later, Farup and his colleagues assessed 25 patients with IBS and 25 healthy controls in a case–control study to evaluate the properties of fecal metabolites as diagnostic biomarkers for IBS. Stool samples were analyzed with gas chromatography for SCFAs and BCFAs (isobutyric and isovaleric acid).
In another case–control study, the fecal metabolites composition and the role of metabolites were investigated in 30 IBS patients with diarrhea (IBS-D) and 15 healthy controls. Data showed that isobutyric acid levels were higher in the stools of patients with IBS, whereas isovalerate levels positively correlated with the severity or frequency of abdominal pain. Both isovaleric and isobutyric acids were associated with visceral hypersensitivity and contributed to abdominal pain. Furthermore, isohexanoate was significantly related to the severity or frequency of abdominal pain in IBS-D patients [
49].
Another team studied the changes in fecal fatty acids after fecal microbiota transplantation (FMT) in IBS (all subtypes) patients. One hundred and forty-two IBS patients were divided into three groups: placebo (own feces), 30 g (superdonor feces), and 60 g (superdonor feces). In responders of the 60 g FMT group, fecal levels of isovaleric and isobutyric rose overall. Isobutyric levels increased in IBS-D and IBS with constipation (IBS-C), but not in IBS mixed (IBS-M) patients.
Moreover, higher concentrations of BCFAs were positively associated with longer colonic transit time (CTT), and longer CTT was also associated with increased proteolytic fermentation in healthy adults, while higher levels of SCFA were related to shorter CTT [
11]. A possible hypothesis is that LFD and higher levels of BCFA could be indicated for IBS-D management, while a high-FODMAP diet and higher SCFA levels could be suitable for IBS-C. Certainly, CTT is affected by a plethora of factors, such as sex, age, stress, body mass index, colonic anatomy, treatment, and gut hormones, among others [
11].
2.2. BCFAs, Inflammation, and Low-FODMAP Diet
2.2.1. Preclinical Trials
In 2019, Tuck et al. [
51] performed experiments in mice with dextran sodium sulfate (DSS)-induced colitis during the inflammatory phase and after treatment. Animals were divided into three groups: two control treatments (“negative-control” and “positive-control”; with and without inflammation, respectively) and a “post-inflammatory” treatment group that mimicked quiescent IBD with IBS-like symptoms. After the recovery, mice were randomized to 2-week low-(0.51 g) or high-FODMAP (4.10 g) diets, respectively. In the positive-control and post-inflammatory treatment groups following the LFD, total levels of stool BCFAs were higher compared with those of the negative controls; statistical significance was reached for isobutyric and isovaleric acid. The results suggested that the higher proteolytic fermentation occurred in the LFD group. Considering the alterations of inflammatory markers, Myeloperoxidase (MPO) activity was lower in the negative-control group, in contrast to its higher levels in the positive-control group, regardless of the FODMAPs content. The research team created a histological score to evaluate the inflammatory status in the colon. The negative controls scored 0, but positive controls had higher scores only in the high-FODMAP diet group. Even if compared with the post-inflammatory group, positive control animals fed with a high-FODMAP diet had significantly higher histological scores. However, no significant differences were observed between low- and high-FODMAP diets. There were no significant changes regarding TNF-α, Granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-10, and IL-4.
The same research team evaluated commercially available rodent diets across research institutions. Forty mice were randomized into four groups to assess the dietary impact on gut microbiota, SCFAs, and BCFAs profiles. Animals in Group A were euthanized at baseline (controls), mice in Group B received the breeding institution chow, mice in Group C received a low-gluten and LFD, and Group D consumed a high-gluten and FODMAP diet. In the LFD group, the BCFA stool levels were higher compared with those in the high-FODMAP group. More specifically, isovalerate and isobutyric acids levels were highest in the LFD group. The explanation is based on protein fermentation and reflects a trend for increased protein metabolism in low-carbohydrate diets.
2.2.2. Clinical Trials
Halmos et al. [53] assessed the effects of an LFD versus a typical Australian diet on fecal biomarkers. This study included 33 participants (27 with IBS and 6 healthy controls). Volunteers followed two diets differing in FODMAP content (LFD contained 3.05 g, whereas the typical Australian diet contained 23.7 g of FODMAPs, respectively). At baseline, isovaleric and isobutyric levels were lower in IBS patients compared to controls, though no difference was noticed during the dietary intervention [53].
Recently, Nordin and her team investigated the effects of different dietary patterns on fecal microbiota, fecal fatty acids, and plasma metabolome in IBS symptoms. One hundred and three IBS patients were randomized into three groups in this double-blinded, placebo-controlled trial. Each group followed all the dietary plans (placebo, gluten, and FODMAP) but in a different sequence. Placebo consisted of 18 g of sucrose, gluten intervention contained 17.3 g of gluten, and the daily dose of FODMAP intake was 50 g. During the intervention, patients filled in questionnaires, and they underwent blood and fecal analyses and anthropometric measurements periodically. Results showed a reduction in plasma levels of isobutyrate in the FODMAP group compared to the placebo, while in feces, a decrease was observed in isovalerate after the gluten diet.
2.3. BCFAs as Potentially Harmful Metabolites
BCFAs have been proposed as markers of colonic protein fermentation, a process that leads to the production of nitrogenous metabolites of protein and amino acid fermentation, such as amines, hydrogen sulfide, p-cresol, phenols, and ammonia. These metabolites are toxic for colonocytes and they are associated with the development inflammatory conditions [
29,
57]. Windey et al. supported, with evidence, the hypothesis that these luminal protein end-products affect not only epithelial cell metabolism but also intestinal barrier function [
58]. In a cross-sectional study, scientists compared the concentrations of BCFAs, ammonia, and fecal pH between vegans and omnivores. Results showed that protein intake was higher in omnivores than in vegans and there was a trend of lower BCFAs concentrations in vegans compared to omnivores, though no significant correlation with protein intake was detected [
57]. Moreover, in an in vitro study [
59], BCFA levels were lower in anaerobic incubation of vegetarians stools compared to incubation of fecal samples of omnivorous. Bacteria from vegetarian donors grew faster on soy protein as substrate, while in omnivorous donors, meat protein and casein were the preferred growth substrates. Fermentation patterns on different substrates were observed between the gut microbiota of vegetarians and omnivore donors. Differences were focused on BCFAs, ammonia, and total bacteria, with lower BCFA levels to be found in vegetarians; this suggests that these donors may have lower branched-chain amino acids intake [
59]. The ability of protein end-products to have harmful effects on the GI is likely related to luminal concentrations of these metabolites. Metabolites’ concentrations may depend upon the balance between the rates of production, detoxification by the colonic epithelium, and absorption or excretion from the large intestine [
25]. The protein source (animal- or plant-based) and the effect of food processing may play a role in altering its digestibility. The proportion of protein consumed and the presence of other nutrients may share a similar efficiency in digestibility and absorption [
26].
3. Conclusions
IBS is a difficult-to-manage disorder, and it is associated with poor health quality of life [
1]. Diet and dietary end-products have been related to the development or the management of the syndrome. While most of the literature focuses on SCFAs, less is known about the way that BCFAs influence the development or dietary management of IBS. LFD is an established dietary intervention for amelioration of IBS symptoms; it has been associated with changes in of the gut microbiome, and due to its composition, it leads to higher protein and amino acid fermentation. Both animal studies and human clinical trials show that changes in the diet of fermentable, indigestible carbohydrates, like in LFD intervention, may lead to production of BCFAs, the byproduct of this fermentation, which, under certain circumstances, might produce large amounts of SCFAs that eventually may modulate and decrease intestinal inflammation [
38].
Although evidence suggests that BCFAs might play a protective role in gut inflammation, other nitrogenous metabolites of protein fermentation, such as amines, hydrogen sulfide, p-cresol, phenols, and ammonia, have detrimental effects on colonocytes and they are associated with gut inflammation, a condition that has been pathogenetically associated with IBS [
1]. Dietary modifications in protein intake by changing, for example, red meat consumption to white meat such as chicken and fish, or plant-based proteins, may reduce the availability of nitrites in the colon [
25].
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms11102387