Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Oral Mucositis, a debilitating side effect of radio and chemotherapy for head and neck cancers, involves inflammation and ulceration of the mucous membranes in the oral cavity. This condition often leads to severe pain, difficulty in eating, and compromised quality of life for cancer patients. The use of natural compounds such as polyphenols has shown promise in preventing and alleviating Oral Mucositis as they possess anti-inflammatory, antioxidant, and healing properties, capable of mitigating the adverse effects of chemo and radiotherapy on the oral mucosa.
- oral mucositis
- polyphenols
- head and neck cancers
- phytocomplex
- curcuminoids
1. Introduction
Head and neck cancers are common tumours, representing approximately 10% of all malignancies in men and 5% in women [1]. They originate in the upper aero-digestive tract at various levels including the larynx, upper trachea, pharynx, and oral and nasal cavity [2]. The onset of these diseases is currently upward and seems to be related to some diffuse risk factors such as consumption of alcohol and tobacco (separately or in combination) and virus infections (e.g., Human Papilloma Virus—HPV) [1][3]. Among the head and neck cancers, oral cancers are remarkably relevant for human health and about 90% of them belong to the squamous cell carcinoma type. In particular, Oral Squamous Cell Carcinoma (OSCC) represents the most frequent malignant tumour affecting the oral mucosal epithelium, with a higher incidence in the male population [4], although a share of OSCC appears to involve an increasingly younger population (under 40). Despite the progress in therapy, the mortality of patients with OSCC has remained steadily high during the last 20 years as compared to other cancers [5]. Its early detection and treatment are still crucial to improve the prognosis, and, in this regard, a combination of multiple therapeutic approaches is frequently recommended to remove cancer and prevent any recurrence [6]. Conventional therapies include surgery, radiotherapy, and chemotherapy; however, these are characterized by several side effects and low patient compliance. Therefore, other more specific treatments such as localized and personalized therapy (which target some genes in cancer cells), administration of natural molecules as adjuvant and chemopreventive agents [7][8], as well as immunotherapy with monoclonal antibodies could be employed [9]. The routine recommendation is surgical management of the primary tumour followed by post-operative radiotherapy or chemoradiotherapy depending on the presence of intermediate/high risk. However, radio- and chemotherapy currently remain the most common therapeutic strategies [10]. Chemotherapy is generally based on drugs such as 5-Fluorouracil (5-FU), Cisplatin, Cetuximab, and Taxanes which being non-selective drugs [11][12] are characterized by several side effects such as gastrointestinal disorders, immune and hematopoietic deficit with consequent infections, inflammation and mucositis in the entire gastrointestinal tract including the oral cavity. On the other hand, head and neck radiotherapy shows unpleased local effects such as severe hyposalivation/xerostomia due to impairment of normal salivary gland function, damage of the oral mucosa, and dermatitis in the overlying epithelium [13]. Major damage to the oral cavity occurs if chemotherapy is associated with radiotherapy as their synergistic effect in the reduction of the epithelial cell turnover leads to thinning of the mucosa itself causing a loss of integrity. Therefore, a compromised function of the epithelium together with a strong inflammation process, oxidative environment, and high susceptibility to bacterial infections often originate in a complex clinical scenario defined as Oral Mucositis (OM) [14]. Considering that oral cancer itself is a disabling disease with a low survival rate, the onset of OM during treatments could further compromise the patient’s overall conditions, lowering their quality of life due to the severe repercussions on nutrition and other mouth functions (as speaking, digesting, or even simply opening the mouth).
2. Oral Mucositis (OM)
OM is a severe acute inflammation affecting the oral mucosa characterized by tissue swelling, ulceration, and erythema [15]. OM develops in around 40–60% of patients with head and neck cancer who receive standard radiotherapy and chemoradiotherapy [16]. OM is described as a painful condition generally resulting in hard discomfort and a negative impact in terms of patients’ quality of life. Depending on the cancer treatment used, it is characterized by a different onset. The common signs that characterize OM are erythema, erosions, and ulcerations of the tissues, which cause a painful condition that can be severe, depending on the size and localization of the damage to the mucous membrane [17][18]. This condition can be further worsened by bacterial infections [4]. In this regard, the World Health Organization (WHO) developed a grading system for the OM severity, ranging from 0 (no OM) to 4 (highly severe OM), which is used to assess its clinical features such as symptoms (e.g., pain), signs (e.g., erythema and ulceration), and oral function (e.g., swallowing ability). This scale also evaluates the severity of clinical manifestations which depends on several factors, such as the type of treatment, the dose, and such individual variables (response to treatment, age, diet, oral hygiene, tumour type, and genetic factors) [19]. Although the onset of OM is the result of different mechanisms that may occur simultaneously, it is possible to identify a common sequence of events that characterizes the pathogenesis of both chemo- and radiotherapy-induced OM. The model described by Sonis in 2004 [20] is useful for the recognition of this sequence of five events:
-
Inflammatory/vascular phase: It is induced by chemotherapy and/or radiotherapy, which induce cytotoxicity in normal cells by directly damaging the DNA and leading to excessive ROS generation. This phenomenon acts as a trigger for the inflammatory process activating different signalling pathways such as proinflammatory cytokines (e.g., IL1 β, IL6, and TNF-α) and prostaglandins [21][22].
-
Activation of transcription factors such as nuclear factor-κ B (NF-κB) and NF-E2-related factor 2 (Nrf2) which can be directly activated by the chemotherapeutic agents, e.g., 5-FU activates the NF-κB, thereby upregulating the genes encoding pro-inflammatory cytokines such as Tumour Necrosis Factor α (TNF-α), interleukin 1β (IL-1β), and IL-6, cyclooxygenase 2 (COX-2), and high-mobility group box 1 protein (HMGB1) or radiation, and indirectly through the ROS release, producing inflammatory mediators, which increase the tissue damage stimulating angiogenesis and vascular permeability [23].
-
Up-regulation and signal amplification stage, leading to loss of the epithelium integrity and, hence, ulcer formation (beginning of OM evolution).
-
Rich inflammatory infiltrate stage, containing macrophages, neutrophils, and mastocytes [24]. In addition, lesions are strongly subjected to bacterial colonization, which contributes to stimulating the innate immune system, thereby increasing the inflammatory response.
-
The final healing phase, characterized by the proliferating and differentiating epithelial cells, leading to the restoration of the integrity of altered mucosa.
Almost 20–40% of cancer patients undergoing conventional chemotherapy manifest this condition (CT-OM) within 4–7 days of treatment, and the clinical signs and symptoms continue for 1 to 2 weeks after.
In head and neck cancer patients, immunotherapy is amongst the most promising strategies. Moreover, the prevalence of related OM seems to be lower compared to traditional chemotherapeutics (methotrexate, cisplatin) [25].
However, OM represents a potential side effect of both targeted therapy and immunotherapy (mammalian target of rapamycin inhibitor-associated stomatitis (mIAS) and immunotherapy-related adverse events (irAEs)) with both severity and clinical presentation depending on the agent used [26]. When combined with conventional chemotherapy, these therapies may increase the risk and severity of mucosal involvement, with a combined presentation of both superficial and deeper, classic Oral Mucositis ulcers [27].
Moreover, Amy et al. reported severe chronic OM associated with pembrolizumab immunotherapy lasting for months even after the drug was stopped and representing the major cause of suffering and eating difficulties for cancer patients [28].
Also, radiation therapy for head and neck cancer causes OM; in this case, side effect onset is delayed, but the duration is longer (between 2 and 6 weeks). Radiation therapy-induced OM (RT-OM) depends on the dose administered, the volume of the tissue treated, and the type of radiation used. The sequelae of CT/RT-OM, including pain, odyno/dysphagia, dysgeusia, decreased oral intake, and local/systemic infection, often require treatment delays, interruptions, and discontinuations which have negative impacts not only on the quality of life but also on tumour control and survival [29].
Currently, the clinical management of OM is mainly aimed at alleviating symptoms, and it is therefore based on the use of conventional drugs suitable for treating pain, inflammation, and infection such as topical anaesthetics (e.g., lidocaine), systemic opioid analgesics (e.g., morphine and fentanyl), and topical and systemic antibiotics and antifungals, which are generally recommended for the prevention of infection [30][31]. Treatment of oral mucosal irAEs is generally carried out with high-potency topical steroids, or systemic steroid/immunosuppressive agents [26].
However, preventing and/or treating any recurrence remains quite complex and often requires multiple approaches which can cause multiple side effects. Therefore, the identification of alternative ways to prevent and treat CT and RT–OM is necessary, especially considering the new targets recently discovered in the pathogenesis of mucosal damage [32].
As the abnormal release of Reactive Oxygen Species (ROS) and the massive inflammatory phase are the main causes of the onset and recurrence of OM, the use of natural antioxidants and anti-inflammatory compounds could be a wise strategy. Among these, polyphenols have recently emerged [33].
Polyphenols in the Prevention and Management of OM
Naturally, plants produce a wide variety of molecules in response to various environmental stimuli (e.g., microbial infection, temperature, etc.). The same compounds, due to their natural actions, could also show interesting properties in humans, useful in the prevention and treatment of several disorders [34][35][36]. Among the wide variety of plant-derived bioactive molecules, polyphenols represent a class of secondary metabolites that are widely distributed in the plant kingdom and are generally synthesized as a defense against such environmental stresses and pathogens [37]. Tea, cocoa powder, grapes, and spices are just a few examples of rich natural sources of polyphenols, but they are also found in edible plants and natural products such as propolis and honey [38].
The large class of polyphenols comprise a broad group of phytochemicals that includes hundreds of natural molecules which are divided into different subclasses according to their main chemical structure. These compounds range from small molecules such as phenolic acids or stilbenes to larger molecules such as tannins [39]. The chemical characteristic that units all of the polyphenols are a phenolic structure based on phenyl ring(s) as a core which presents one or more hydroxyl substituents, essential for the radical scavenging activity (due to electron delocalization and stabilization of the formed radical) [37]. According to their structure polyphenols can be classified into two main groups: flavonoids and non-flavonoids (Figure 1). Flavonoids possess two phenyl rings fused with a central heterocyclic ring containing oxygen [40]. They can be further subdivided into flavonols, flavones, flavanones, flavanols isoflavones, anthocyanidins, and chalcones.
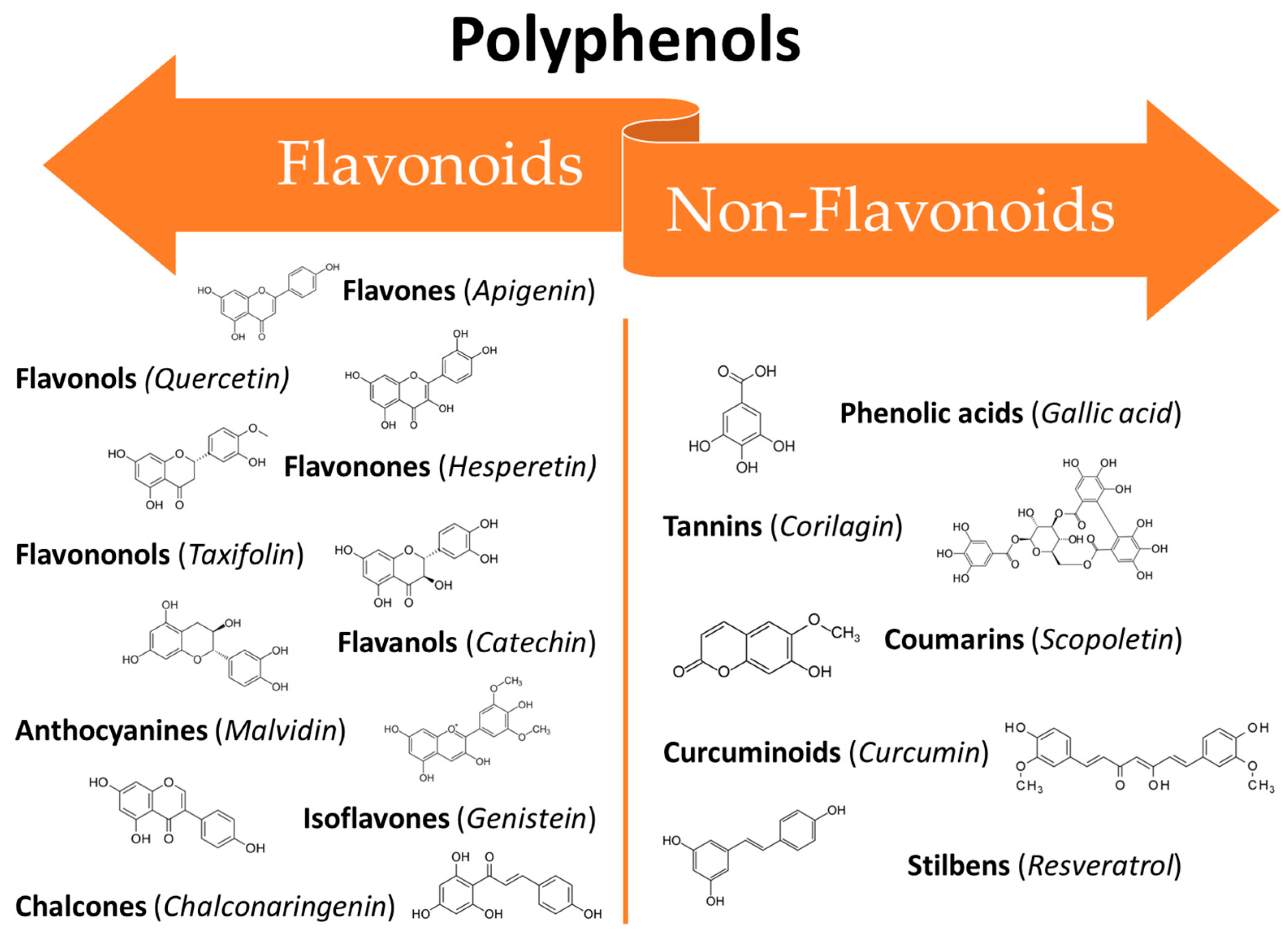
Figure 1. Classification of polyphenols: main classes and subclasses with some representative molecules.
On the other hand, the non-flavonoids can be divided into different subclasses such as phenolic acids, tannins, coumarins, curcuminoids, and stilbenes [41]. In recent years, polyphenols have gained the attention of researchers all around the world thanks to their broad spectrum of bioactive properties, which may be useful in improving human health, as well as their low side effects. They can interact with different cellular pathways, enzymatic mechanisms, and hormones controlling gene expression [42]. Thus, the mechanisms involved in their activity are complex and often still controversial. Their wide range of biological activities includes antioxidant [38], antifungal [43][44], anti-inflammatory [45], anti-aging [46], osteogenic [47], chemopreventive [48], and antitumoural [49] properties which makes them potentially useful in several fields ranging from pharmaceuticals to cosmetics [50]. Several in vitro studies, in vivo studies, and clinical trials have been conducted to assess the efficacy of polyphenols against the oral mucosa damage that occurs as a side effect of anticancer therapies. The broad-spectrum activities of polyphenols make them ideal candidates for clinical use in many areas of medicine. However, their use is still limited due to their unfavourable physicochemical properties such as low water solubility, instability at high temperatures and alkaline pH, as well as a massive first-pass effect after oral administration, resulting in extremely low bioavailability [51]. Recently, researchers have proposed several innovative solutions to mitigate these drawbacks in order to benefit from the therapeutic effects of these potent molecules. Some of the most recent innovations are related to new techniques for their extraction and manipulation and the design of innovative drug delivery platforms and systems suitable for minimizing the degradation phenomena and improving bioavailability [51].
3. Curcuminoids
Curcuminoids are the main active components of the rhizomes of Curcuma longa L., an herb widely used as a traditional remedy in China and Southeast Asia. The term “curcuminoids” indicates a mixture of compounds such as curcumin, Dimethoxy curcumin, and Bis-dimethoxy curcumin. Furthermore, there is also a fourth molecule called Cyclocurcumin, which was initially identified as curcuminoid but was later considered only to be a structural isomer of curcumin [52]. Curcuminoids are composed of a core structure having two aromatic benzene methoxy rings linked by an unsaturated seven-carbon chain consisting of an α,β-unsaturated β-diketone. The presence of this specific group ensures the pH-dependent keto-enol tautomerism [53]. This keto-enol balance is very important for the physico-chemical and antioxidant properties of curcuminoids. Considering curcumin as an example, when it is in the enolic form both aromatic rings can interact, delocalizing the electrons present in the π orbitals. The chemical structure of curcuminoids also permits a wide range of beneficial and therapeutic effects as antimicrobial [54], anti-inflammatory [55][56], neuroprotective [57], and anticancer [58].
Curcumin (CUR, Figure 2), the most studied of the curcuminoids, exerts its anti-inflammatory effects through upregulation of the peroxisome proliferator-activated receptor gamma (PPAR-γ) [59] and downregulation of NF-Kβ, thereby suppressing the subsequent synthesis of cytokines such as TNF-α, IL-1β, IL-6 and IL-8 and vascular endothelial growth factor (VEGF) [60]. It increases the plasma levels of superoxide dismutase (SOD) and glutathione peroxidase, enhancing the catalase activity and reducing the plasma levels of lipid peroxidase. CUR is able to participate in many signalling pathways by modulating several signalling molecules (e.g., pro-apoptotic proteins, COX-2, C-reactive protein, prostaglandin E2) and adhesion molecules [61].
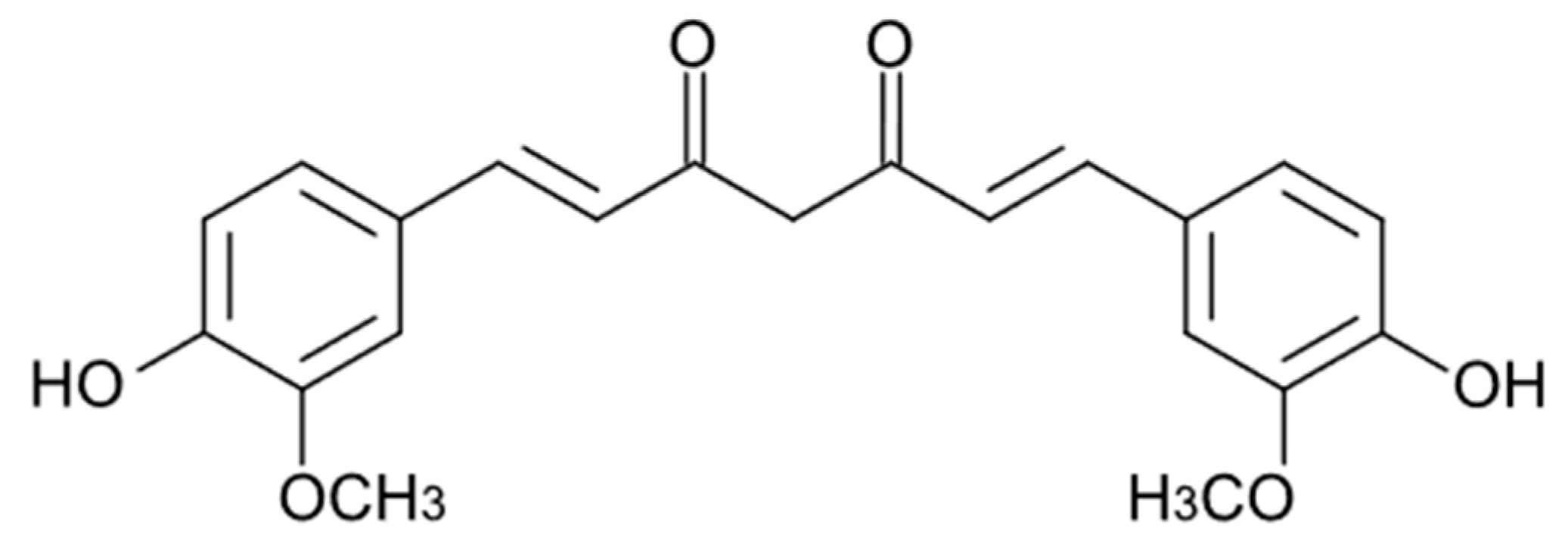
Figure 2. Chemical structure of curcumin.
The liphophilic-like structure confers to CUR a very low bioavailability after oral intake. Indeed, in healthy human subjects, after oral ingestion of 10 g, CUR showed an area under the curve (AUC) of 35.33 ± 3.78 μg/mL × hr and a Cmax of 2.30 ± 0.26 μg/mL, with a half-life (T1/2) of 6.77 ± 0.83 hr. In all subjects, CUR was detected in plasma primarily as glucuronide and sulfate conjugates, rarely as free CUR. Notably, no subjects reported mild or severe side effects [62].
3.1. In Vivo Animal Studies
The ability of curcuminoids to accelerate the resolution of inflammation and the reepithelization of the buccal mucosa lesions is enlightened in a prospective randomized controlled–blinded in vivo study performed by Schmidt et al. [63] in 2019. This study included 72 male golden Syrian hamsters affected by 5-FU induced OM and evaluated the effects of a topical treatment with (i) a mucoadhesive formulation containing curcuminoids (indicated as MCF and consisting of mucoadhesive gel containing CUR 20 mg/mL), (ii) a Chamomilla recutita L. fluid extract (indicated as Ad-Muc®), used as a phytotherapeutic positive control, and (iii) a placebo, corresponding to the CUR-free mucoadhesive formulation, each administered twice daily (0.5 g/dose) for 2 weeks. The treated groups (including the placebo) were compared with a further control group, which received 5-FU alone to induce OM and was then treated twice daily under identical conditions, but no substances were applied. The collected histopathological and macroscopic analyses showed that both MCF and Ad-Muc® enhanced the tissue healing processes through the formation of new epithelial tissue thus covering the entire thickness of wounds. Moreover, the reepithelization process occurred rapidly as a result of the therapy. The so-treated hamster also exhibited lower angiogenesis, vascularization, and TGF-β1-labelling than the control group (animal treated with 5-FU alone) and the placebo group.
In 2022, Dvoretskiy et al. [64] evaluated the efficacy of polyphenols and nutrient topical administration in reducing radiotherapy-induced OM in Syrian golden hamsters. Eighty 5–6-weeks-old animals were subjected to a single dose of radiation (40 Gy) on day 0, resulting in OM after 6 days and achieving a peak (moderate or severe OM, WHO score ≥3) at days 14–16. Animals were evaluated up to 28 days and treated topically from day 1 to day 20, as follows: (i) receiving 0.25 mL of CUR 50 μg/mL in a 2% (v/v) ethanol in water solution; receiving 0.25 mL of CUR 100 μg/mL in a 2% (v/v) ethanol in water solution; (iii) control 1 receiving 0.25 mL of a 2% (v/v) ethanol in water solution; (iv) receiving 0.25 mL of Quercetin (QRC) 50 μg/mL in a 2% (v/v) DMSO in water solution; (v) receiving 0.25 mL of QRC 100 μg/mL in a 2% (v/v) DMSO in water solution; vi) control 2 receiving 0.2 mL of a 2% (v/v) DMSO in water solution; (vii) control 3 receiving 0.25 mL of water; (viii) receiving 0.2 mL of alanyl-glutamine dipeptide 30 mg/mL in water; (ix) receiving 0.2 mL of Arg/Gln/HMB 50 mg/mL in water; and (x) receiving 0.2 mL of Arg/Gln/HMB 100 mg/mL in water. With regard to CUR effectiveness, it is first remarkable that the animals in the control ethanol group experienced severe OM (score ≥3) for 35.5% of the experimental days, whereas this value decreased to 17.6% in the CUR 50 group. The OM score in the CUR 50 group was statistically reduced compared to the control from day 10, while the CUR 100 treatment showed significant results only after 28 days. According to the Mann–Whitney test, only CUR 50 was effective as it achieved almost 2 days of significant reduction during the period of study. In contrast, the amino acids-based treatments did not display the expected benefit probably due to low dosage.
3.2. Clinical Trials
The role of curcumin and curcuminoids in restoring buccal tissue in OM injuries induced by radio- and chemo-therapies is well established in the literature. Indeed, Normando et al. [65] published a systematic review in 2019 with the aim of evaluating the effects of turmeric and curcumin in the management of chemo- and radiotherapy-induced OM. In addition, the literature already collected by these colleagues will not be reported here again, even if several papers should meet the inclusion criteria here selected. Consequently, only the newest papers are reported below. It is relevant to emphasize that even if the considered time span here is limited (the last 4 years), several papers reporting on clinical trials continue to prove the usefulness of curcuminoids to prevent and treat chemo- and radiotherapy-induced OM. In 2020, Shah et al. [66] conducted a triple-blind clinical trial to assess the effectiveness and safety of a mouthwash containing CUR 0.1% (w/v), compared to a commercial one containing benzydamine 0.15% (w/v) (COOLORA™) for the treatment of radiotherapy-induced OM. Seventy-four patients with conclusive OSCC and undergoing radiotherapy (60–70 Gy) were divided into two groups receiving each 10 mL of CUR-containing mouthwash or the commercial formulation (control group) three times daily for 6 weeks. Patients were evaluated once a week and assigned an OM score (ranging from 0 to 4). Both the preventive and the curative effects of the treatment were assessed by evaluating the OM score. In particular, a score ≥1 was defined as the onset of OM, while a score ≤ 2 was defined as tolerable mucositis and ≥ 3 as intolerable mucositis. There were no significant differences between the two groups in terms of healing effect as both formulations were effective in the treatment of OM. However, CUR emerged as a more effective preventive strategy, as the use of the CUR-containing mouthwash reduced the risk of OM onset in almost 50% of patients and also showed a promising delay in the OM onset of 2 weeks. Furthermore, at the end of this clinical trial, there were no subjects in the CUR group characterized by an OM score ≥ 3.
In 2022, de Cássia Dias Viana Andrade et al. [67] investigated the role of both photobiomodulation and antimicrobial photodynamic therapy mediated by CUR and blue LED as an adjuvant strategy to manage OM in patients undergoing chemo- or radiotherapy. Thirty patients, with stable oral mucosa lesions and treated by radio- or chemotherapy, were randomly divided into three groups: (i) the control group receiving nystatin as standard treatment protocol; (ii) the group treated with low-level laser (λ = 660 nm, power: 100 mW) 3 times a week for 1 month; and (iii) the group treated with 450 nm blue LED and CUR (7.5 mg in 10 mL, prepared at the time of use) as photosensitizer once a week, for 1 month each. Patients were evaluated weekly for OM score and Candida albicans infection. Results showed that the participants who received the photo-treatments both in the presence and in the absence of CUR, showed a long-term (after 21 or 30 days of therapy) reduction of C. albicans infection, pain, and OM score (from the 21st day of treatment) when compared to the control group (which worsened after the 14th day and for the entire duration of the experiment). In particular, the CUR-based option was more effective than the photo-treatment alone in both minimizing infections and reducing OM symptoms. A double-blind clinical trial comparing the effect of three formulations loaded with mucosamin, chlorhexidine, and CUR in the treatment of OM was performed by Fardad et al. [68] in 2023. Specifically, 82 patients undergoing chemotherapy were divided into three groups treated with (i) commercially available Mucosamin® oral spray (Professional Dietetics®, Milan, Italy) four puffs/day for two weeks; (ii) commercially available 0.2% (w/v) chlorhexidine mouthwash (Behsa®, Tehran, Iran) in 1:1 dilution for 1 min four times/day for two weeks; and (iii) 0.5% (w/v) CUR-loaded gel, four times/day for two weeks. Patients were assessed daily using the WHO score (0–4 scale) and OMAS scores (0–2 scale for erythema intensity; 0–3 scale for ulceration). Results showed that the severity of OM, as measured by WHO score, was significantly reduced in patients subjected to the CUR-based treatment compared to the other groups, with a complete restoration of the lesions after 4 days. On the other end, the other two approaches did not lead to complete recovery in the time period considered. CUR was also superior in terms of the OMAS score: the erythema disappeared after 4 days, compared to the 6 days required for the mucosamin-based treatment, while chlorhexidine did not resolve this symptom. Moreover, the ulcerative section parameter confirmed the complete tissue restoration after 5 days of treatment in the CUR group (tissue restoration was almost complete after 4 days), whereas mucosamin required 11 days of therapy and chlorhexidine was not fully effective again. In conclusion, all the formulations were found to be useful in the management the OM symptoms, but the use of CUR resulted in extremely rapid effects.
Another recent example of the efficacy of turmeric in the treatment of OM is the study published by Soni et al. [69] in 2022. A double-blind clinical trial was performed on sixty patients undergoing chemotherapy. Patients were divided into three groups: (i) low dose of bioenhanced turmeric formulation containing curcuminoids and essential oil of turmeric (1 g/die, per os); (ii) high dose of bioenhanced turmeric formulation (1.5 g/die, per os); and (iii) placebo, for a total of 6 weeks with concurrent chemotherapy. OM severity, dysphagia, pain, dermatitis, and weight loss were the considered parameters evaluated during the study. Results highlighted that OM severity (grade 3) and OM-associated symptoms were significantly lower in both the two turmeric-treated groups. Additionally, the 1.5 g/die dose therapy was slightly more effective than the 1 g/die therapy, thus suggesting a real dose dependence. Furthermore, the groups treated with curcuminoids were more compliant, lost less weight, and did not require hospitalization compared to the placebo group. Due to the unfavourable physico-chemical properties of curcuminoids, their use in human studies could be limited in terms of the choice of formulation to be administered. As a result, the last 3 clinical studies reported below will all employ a nanostructured CUR formulation. The latter is SinaCurcumin®, a registered nanomicellar formulation designed for oral use containing curcumin, bisdemethoxycurcumin, and desmethoxycurcumin. This product was developed by the Nanotechnology Research Center of Mashhad University of Medical Science and marketed by Exir Nano Sina Company in Tehran, Iran. Each softgel capsule contains 80 mg of curcumin as nanomicelles having a CUR loading efficacy of almost 100% and significantly higher bioavailability after oral intake than the simple curcumin powder form [70][71]. A double-blind clinical trial was conducted by Delavarian et al. [72] in 2019 to assess the effects of SinaCurcumin® on radiotherapy-induced OM. Thirty-two patients undergoing radiotherapy (50 Gy) were equally divided into study and control groups receiving SinaCurcumin® capsules and lactose-loaded placebo tablets, respectively, once a day for 6 weeks. As observed, the study group displayed a one-week delay in the OM onset compared to the control group. However, once OM manifested, its severity gradually increased over time in both groups throughout the radiotherapy treatment in a time-dependent manner. Nevertheless, the beneficial effects of SinaCurcumin® were undoubtedly confirmed as the study group showed less weight loss, probably related to the ameliorative effect of CUR leading to better food intake, and no grade 4 OM was registered. In contrast, almost 50% of patients in the control group manifested the highest WHO grade of mucositis.
Kia et al. [73] also evaluated the effectiveness of SinaCurcumin® in 2021. Fifty patients undergoing chemotherapy (30–50 mg Cisplatin or 640–750 mg 5-FU) and/or radiotherapy (60–70 Gy) to treat head and neck cancers were selected and divided into two groups: the treated group received SinaCurcumin® and the control one received placebo capsules, both twice daily for 7 weeks. The severity of mucositis and pain scores were evaluated after 1, 4, and 7 weeks of treatment. It was shown that OM was significantly more severe in the control group than in the treated group for the entire duration of the trial, while SinaCurcumin® reduced the pain only at the end of the experiment. Furthermore, as expected, patients who received both chemo- and radiotherapy experienced a worsening of the OM clinical signs compared to the patients who underwent chemotherapy alone. CUR-based treatment was then confirmed to be effective in decreasing the severity and progression of OM, while also ameliorating patients’ quality of life, especially for those subjected exclusively to chemotherapy. The efficacy of SinaCurcumin® was further demonstrated by Ramezani et al. [74] in 2023 through a randomized clinical trial comparing the latter oral dosage form with a CUR-loaded mouthwash. Forty-five patients affected by radiotherapy-induced OM (severity 1–3 in the WHO scale) and still ongoing radiotherapy were divided into three groups receiving (i) 40 mg/die of SinaCurcumin®, (ii) CUR mouthwash 0.1% (w/v), or (iii) placebo mouthwash, both liquid dosage forms for 1 min, three times daily. After 21 days, all the patients treated with CUR both topically and orally showed a reduction in OM severity, signs, and symptoms compared to the placebo group. In addition, between 15 and 33% of the subjects in the CUR-treated groups showed a complete resolution of the ulcer, while patients in the placebo group still had OM at the end of the trial. However, no statistical differences in the efficacy of the two CUR-loaded formulations were observed, probably due to the limited number of participants involved in the study as a result of the COVID-19 pandemic.
This entry is adapted from the peer-reviewed paper 10.3390/cancers16020260
References
- Vigneswaran, N.; Williams, M.D. Epidemiologic Trends in Head and Neck Cancer and Aids in Diagnosis. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 123–141.
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and Neck Cancer. Lancet 2008, 371, 1695–1709.
- Panzarella, V.; Campisi, G.; Giardina, Y.; Maniscalco, L.; Capra, G.; Rodolico, V.; Di Fede, O.; Mauceri, R. Low Frequency of Human Papillomavirus in Strictly Site-coded Oral Squamous Cell Carcinomas, Using the Latest Nhi/Seer-icd Systems: A Pilot Observational Study and Critical Review. Cancers 2021, 13, 4595.
- Singh, V.; Singh, A. Oral Mucositis. Natl. J. Maxillofac. Surg. 2020, 11, 159.
- Mauceri, R.; Bazzano, M.; Coppini, M.; Tozzo, P.; Panzarella, V.; Campisi, G. Diagnostic Delay of Oral Squamous Cell Carcinoma and the Fear of Diagnosis: A Scoping Review. Front. Psychol. 2022, 13, 1009080.
- Singh, M.P.; Kumar, V.; Agarwal, A.; Kumar, R.; Bhatt, M.L.B.; Misra, S. Clinico-Epidemiological Study of Oral Squamous Cell Carcinoma: A Tertiary Care Centre Study in North India. J. Oral Biol. Craniofac. Res. 2016, 6, 32–35.
- Di Prima, G.; Conigliaro, A.; De Caro, V. Mucoadhesive Polymeric Films to Enhance Barbaloin Penetration into Buccal Mucosa: A Novel Approach to Chemoprevention. AAPS PharmSciTech 2019, 20, 18.
- De Caro, V.; Scaturro, A.L.; Di Prima, G.; Avellone, G.; Sutera, F.M.; Di Fede, O.; Campisi, G.; Giannola, L.I. Aloin Delivery on Buccal Mucosa: Ex Vivo Studies and Design of a New Locoregional Dosing System. Drug Dev. Ind. Pharm. 2015, 41, 1541–1547.
- Tamimi, A.; Tamimi, A.; Sorkheh, F.; Asl, S.M.; Ghafari, A.; Karimi, A.G.; Erabi, G.; Pourmontaseri, H.; Deravi, N. Monoclonal Antibodies for the Treatment of Squamous Cell Carcinoma: A Literature Review. Cancer Rep. 2023, 6, e1802.
- Budach, W.; Hehr, T.; Budach, V.; Belka, C.; Dietz, K. A Meta-Analysis of Hyperfractionated and Accelerated Radiotherapy and Combined Chemotherapy and Radiotherapy Regimens in Unresected Locally Advanced Squamous Cell Carcinoma of the Head and Neck. BMC Cancer 2006, 6, 28.
- Hanemaaijer, S.H.; Kok, I.C.; Fehrmann, R.S.N.; van der Vegt, B.; Gietema, J.A.; Plaat, B.E.C.; van Vugt, M.A.T.M.; Vergeer, M.R.; Leemans, C.R.; Langendijk, J.A.; et al. Comparison of Carboplatin With 5-Fluorouracil vs. Cisplatin as Concomitant Chemoradiotherapy for Locally Advanced Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 761.
- Guigay, J.; Tahara, M.; Licitra, L.; Keilholz, U.; Friesland, S.; Witzler, P.; Mesía, R. The Evolving Role of Taxanes in Combination with Cetuximab for the Treatment of Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck: Evidence, Advantages, and Future Directions. Front. Oncol. 2019, 9, 668.
- Pinna, R.; Campus, G.; Cumbo, E.; Mura, I.; Milia, E. Xerostomia Induced by Radiotherapy: An Overview of the Physiopathology, Clinical Evidence, and Management of the Oral Damage. Ther. Clin. Risk Manag. 2015, 11, 171–188.
- Raber-Durlacher, J.E.; Elad, S.; Barasch, A. Oral Mucositis. Oral Oncol. 2010, 46, 452–456.
- Rodríguez-Caballero, A.; Torres-Lagares, D.; Robles-García, M.; Pachón-Ibáñez, J.; González-Padilla, D.; Gutiérrez-Pérez, J.L. Cancer Treatment-Induced Oral Mucositis: A Critical Review. Int. J. Oral Maxillofac. Surg. 2012, 41, 225–238.
- Pulito, C.; Cristaudo, A.; La Porta, C.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral Mucositis: The Hidden Side of Cancer Therapy. J. Exp. Clin. Cancer Res. 2020, 39, 1–15.
- Beech, N.; Robinson, S.; Porceddu, S.; Batstone, M. Dental Management of Patients Irradiated for Head and neck Cancer. Aust. Dent. J. 2014, 59, 20–28.
- Volpato, L.E.R.; Silva, T.C.; Oliveira, T.M.; Sakai, V.T.; Machado, M.A.A.M. Radiation Therapy and Chemotherapy-Induced Oral Mucositis. Braz. J. Otorhinolaryngol. 2007, 73, 562–568.
- Oronsky, B.; Goyal, S.; Kim, M.M.; Cabrales, P.; Lybeck, M.; Caroen, S.; Oronsky, N.; Burbano, E.; Carter, C.; Oronsky, A. A Review of Clinical Radioprotection and Chemoprotection for Oral Mucositis. Transl. Oncol. 2018, 11, 771–778.
- Sonis, S.T. The Pathobiology of Mucositis. Nat. Rev. Cancer 2004, 4, 277–284.
- Sonis, S.T. Mucositis: The Impact, Biology and Therapeutic Opportunities of Oral Mucositis. Oral Oncol. 2009, 45, 1015–1020.
- Siomek, A.; Tujakowski, J.; Gackowski, D.; Rozalski, R.; Foksinski, M.; Dziaman, T.; Roszkowski, K.; Olinski, R. Severe Oxidatively Damaged DNA after Cisplatin Treatment of Cancer Patients. Int. J. Cancer 2006, 119, 2228–2230.
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 System in Development, Oxidative Stress Response and Diseases: An Evolutionarily Conserved Mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247.
- Worthington, H.V.; Clarkson, J.E.; Bryan, G.; Furness, S.; Glenny, A.M.; Littlewood, A.; McCabe, M.G.; Meyer, S.; Khalid, T.; Riley, P. Interventions for Preventing Oral Mucositis for Patients with Cancer Receiving Treatment. Cochrane Database Syst. Rev. 2011, 2021, CD000978.
- Peña-Cardelles, J.F.; Salgado-Peralvo, A.O.; Garrido-Martínez, P.; Carretero, J.L.C.; Pozo-Kreilinger, J.J.; Moro-Rodríguez, J.E. Oral Mucositis. Is It Present in the Immunotherapy of the Immune Checkpoint Pd1/Pd-L1 against Oral Cancer? A Systematic Review. Med. Oral Patol. Oral Cir. Bucal. 2021, 26, e494–e501.
- Villa, A.; Kuten-Shorrer, M. Pathogenesis of Oral Toxicities Associated with Targeted Therapy and Immunotherapy. Int. J. Mol. Sci. 2023, 24, 8188.
- Miroddi, M.; Sterrantino, C.; Simonelli, I.; Ciminata, G.; Phillips, R.S.; Calapai, G. Risk of Grade 3-4 Diarrhea and Mucositis in Colorectal Cancer Patients Receiving Anti-EGFR Monoclonal Antibodies Regimens: A Meta-Analysis of 18 Randomized Controlled Clinical Trials. Crit. Rev. Oncol. Hematol. 2015, 96, 355–371.
- Amy, D.P.B.; Shalabi, A.; Finfter, O.; Birenzweig, Y.; Zadik, Y. Severe Chronic Nonlichenoid Oral Mucositis in Pembrolizumab-Treated Patients: New Cases and a Review of the Literature. Immunotherapy 2020, 12, 777–784.
- Lalla, R.V.; Treister, N.; Sollecito, T.; Schmidt, B.; Patton, L.L.; Mohammadi, K.; Hodges, J.S.; Brennan, M.T. Oral Complications at 6 Months after Radiation Therapy for Head and Neck Cancer. Oral Dis. 2017, 23, 1134–1143.
- Saunders, D.P.; Rouleau, T.; Cheng, K.; Yarom, N.; Kandwal, A.; Joy, J.; Bektas Kayhan, K.; van de Wetering, M.; Brito-Dellan, N.; Kataoka, T.; et al. Systematic Review of Antimicrobials, Mucosal Coating Agents, Anesthetics, and Analgesics for the Management of Oral Mucositis in Cancer Patients and Clinical Practice Guidelines. Support Care Cancer 2020, 28, 2473–2484.
- Yarom, N.; Hovan, A.; Bossi, P.; Ariyawardana, A.; Jensen, S.B.; Gobbo, M.; Saca-Hazboun, H.; Kandwal, A.; Majorana, A.; Ottaviani, G.; et al. Systematic Review of Natural and Miscellaneous Agents, for the Management of Oral Mucositis in Cancer Patients and Clinical Practice Guidelines—Part 2: Honey, Herbal Compounds, Saliva Stimulants, Probiotics, and Miscellaneous Agents. Support Care Cancer 2020, 28, 2457–2472.
- Bowen, J.; Al-Dasooqi, N.; Bossi, P.; Wardill, H.; Van Sebille, Y.; Al-Azri, A.; Bateman, E.; Correa, M.E.; Raber-Durlacher, J.; Kandwal, A.; et al. The Pathogenesis of Mucositis: Updated Perspectives and Emerging Targets. Support Care Cancer 2019, 27, 4023–4033.
- Varoni, E.M.; Lodi, G.; Sardella, A.; Carrassi, A.; Iriti, M. Plant Polyphenols and Oral Health: Old Phytochemicals for New Fields. Curr. Med. Chem. 2012, 19, 1706–1720.
- Rahman, M.M.; Wang, X.; Islam, M.R.; Akash, S.; Supti, F.A.; Mitu, M.I.; Harun-Or-Rashid, M.; Aktar, M.N.; Khatun Kali, M.S.; Jahan, F.I.; et al. Multifunctional Role of Natural Products for the Treatment of Parkinson’s Disease: At a Glance. Front. Pharmacol. 2022, 13, 976385.
- Choudhury, H.; Pandey, M.; Hua, C.K.; Mun, C.S.; Jing, J.K.; Kong, L.; Ern, L.Y.; Ashraf, N.A.; Kit, S.W.; Yee, T.S.; et al. An Update on Natural Compounds in the Remedy of Diabetes Mellitus: A Systematic Review. J. Tradit. Complement. Med. 2018, 8, 361–376.
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374.
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From Theory to Practice. Foods 2021, 10, 2595.
- Rudrapal, M.; Khairnar, S.J.; Khan, J.; Dukhyil, A.B.; Ansari, M.A.; Alomary, M.N.; Alshabrmi, F.M.; Palai, S.; Deb, P.K.; Devi, R. Dietary Polyphenols and Their Role in Oxidative Stress-Induced Human Diseases: Insights into Protective Effects, Antioxidant Potentials and Mechanism(s) of Action. Front. Pharmacol. 2022, 13, 806470.
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246.
- Dias, M.C.; Pinto, D.C.G.A.; Silva, A.M.S. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377.
- Vauzour, D. Effect of Flavonoids on Learning, Memory and Neurocognitive Performance: Relevance and Potential Implications for Alzheimer’s Disease Pathophysiology. J. Sci. Food Agric. 2014, 94, 1042–1056.
- Panickar, K.S. Effects of Dietary Polyphenols on Neuroregulatory Factors and Pathways That Mediate Food Intake and Energy Regulation in Obesity. Mol. Nutr. Food Res. 2013, 57, 34–47.
- Sitheeque, M.A.M.; Panagoda, G.J.; Yau, J.; Amarakoon, A.M.T.; Udagama, U.R.N.; Samaranayake, L.P. Antifungal Activity of Black Tea Polyphenols (Catechins and Theaflavins) against Candida Species. Chemotherapy 2009, 55, 189–196.
- Simonetti, G.; Brasili, E.; Pasqua, G. Antifungal Activity of Phenolic and Polyphenolic Compounds from Different Matrices of Vitis vinifera L. Against Human Pathogens. Molecules 2020, 25, 3748.
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and in Vivo Studies. Antioxidants 2020, 9, 35.
- Luo, J.; Si, H.; Jia, Z.; Liu, D. Dietary Anti-Aging Polyphenols and Potential Mechanisms. Antioxidants 2021, 10, 283.
- Shanmugavadivu, A.; Balagangadharan, K.; Selvamurugan, N. Angiogenic and Osteogenic Effects of Flavonoids in Bone Regeneration. Biotechnol. Bioeng. 2022, 119, 2313–2330.
- Angellotti, G.; Di Prima, G.; Belfiore, E.; Campisi, G.; De Caro, V. Chemopreventive and Anticancer Role of Resveratrol against Oral Squamous Cell Carcinoma. Pharmaceutics 2023, 15, 275.
- do Carmo, M.A.V.; Pressete, C.G.; Marques, M.J.; Granato, D.; Azevedo, L. Polyphenols as Potential Antiproliferative Agents: Scientific Trends. Curr. Opin. Food Sci. 2018, 24, 26–35.
- Di Prima, G.; Belfiore, E.; Migliore, M.; Scarpaci, A.G.; Angellotti, G.; Restivo, I.; Allegra, M.; Arizza, V.; De Caro, V. Green Extraction of Polyphenols from Waste Bentonite to Produce Functional Antioxidant Excipients for Cosmetic and Pharmaceutical Purposes: A Waste-to-Market Approach. Antioxidants 2022, 11, 2493.
- Lewandowska, U.; Szewczyk, K.; Hrabec, E.; Janecka, A.; Gorlach, S. Overview of Metabolism and Bioavailability Enhancement of Polyphenols. J. Agric. Food Chem. 2013, 61, 12183–12199.
- Mukherjee, S.; Kar, S.K. Curcuminoids: The Novel Molecules of Nature. In Herbs and Spices. New Processing Technologies; IntechOpen: London, UK, 2021.
- Priyadarsini, K.I. Photophysics, Photochemistry and Photobiology of Curcumin: Studies from Organic Solutions, Bio-Mimetics and Living Cells. J. Photochem. Photobiol. C Photochem. 2009, 10, 81–95.
- Lüer, S.; Troller, R.; Jetter, M.; Spaniol, V.; Aebi, C. Topical Curcumin Can Inhibit Deleterious Effects of Upper Respiratory Tract Bacteria on Human Oropharyngeal Cells in Vitro: Potential Role for Patients with Cancer Therapy Induced Mucositis? Support Care Cancer 2011, 19, 799–806.
- Shimizu, K.; Funamoto, M.; Sunagawa, Y.; Shimizu, S.; Katanasaka, Y.; Miyazaki, Y.; Wada, H.; Hasegawa, K.; Morimoto, T. Anti-Inflammatory Action of Curcumin and Its Use in the Treatment of Lifestyle-Related Diseases. Eur. Cardiol. Rev. 2019, 14, 117–122.
- Amalraj, A.; Varma, K.; Jacob, J.; Divya, C.; Kunnumakkara, A.B.; Stohs, S.J.; Gopi, S. A Novel Highly Bioavailable Curcumin Formulation Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients: A Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study. J. Med. Food 2017, 20, 1022–1030.
- Dohare, P.; Garg, P.; Sharma, U.; Jagannathan, N.R.; Ray, M. Neuroprotective Efficacy and Therapeutic Window of Curcuma Oil: In Rat Embolic Stroke Model. BMC Complement. Altern. Med. 2008, 8, 55.
- Tomeh, M.A.; Hadianamrei, R.; Zhao, X. A Review of Curcumin and Its Derivatives as Anticancer Agents. Int. J. Mol. Sci. 2019, 20, 1033.
- Jacob, A.; Wu, R.; Zhou, M.; Wang, P. Mechanism of the Anti-Inflammatory Effect of Curcumin: PPAR-γ Activation. PPAR Res. 2007, 2007, 89369.
- Binion, D.G.; Otterson, M.F.; Rafiee, P. Curcumin Inhibits VEGF-Mediated Angiogenesis in Human Intestinal Microvascular Endothelial Cells through COX-2 and MAPK Inhibition. Gut 2008, 57, 1509–1517.
- Lüer, S.; Troller, R.; Aebi, C. Antibacterial and Antiinflammatory Kinetics of Curcumin as a Potential Antimucositis Agent in Cancer Patients. Nutr. Cancer 2012, 64, 975–981.
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of Curcumin Conjugate Metabolites in Healthy Human Subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1411–1417.
- Schmidt, T.R.; Curra, M.; Wagner, V.P.; Martins, M.A.T.; de Oliveira, A.C.; Batista, A.C.; Valadares, M.C.; Marreto, R.N.; Martins, M.D. Mucoadhesive Formulation Containing Curcuma longa L. Reduces Oral Mucositis Induced by 5-Fluorouracil in Hamsters. Phytother. Res. 2019, 33, 881–890.
- Dvoretskiy, S.; Pereira, S.L.; Das, T. Efficacy of Nutrients in Reducing the Symptoms of Radiation Induced Oral Mucositis in a Hamster Model. Nutr. Cancer 2022, 74, 1079–1089.
- Normando, A.G.C.; de Menêses, A.G.; de Toledo, I.P.; Borges, G.Á.; de Lima, C.L.; dos Reis, P.E.D.; Guerra, E.N.S. Effects of Turmeric and Curcumin on Oral Mucositis: A Systematic Review. Phytother. Res. 2019, 33, 1318–1329.
- Shah, S.; Rath, H.; Sharma, G.; Senapati, S.N.; Mishra, E. Effectiveness of Curcumin Mouthwash on Radiation-Induced Oral Mucositis among Head and Neck Cancer Patients: A Triple-Blind, Pilot Randomised Controlled Trial. Indian J. Dent. Res. 2020, 31, 718–727.
- de Cássia Dias Viana Andrade, R.; Azevedo Reis, T.; Rosa, L.P.; de Oliveira Santos, G.P.; da CristinaSilva, F. Comparative Randomized Trial Study about the Efficacy of Photobiomodulation and Curcumin Antimicrobial Photodynamic Therapy as a Coadjuvant Treatment of Oral Mucositis in Oncologic Patients: Antimicrobial, Analgesic, and Degree Alteration Effect. Support Care Cancer 2022, 30, 7365–7371.
- Fardad, F.; Ghasemi, K.; Ansarinejad, N.; Khodakarim, N.; Nasiripour, S.; Farasatinasab, M. A Comparative Study to Assess the Effectiveness of Curcumin, Mucosamin, and Chlorhexidine in Chemotherapy-Induced Oral Mucositis. Explore 2023, 19, 65–70.
- Soni, T.P.; Gupta, A.K.; Sharma, L.M.; Singhal, H.; Sharma, S.; Gothwal, R.S. A Randomized, Placebo-Controlled Study to Evaluate the Effect of Bio-Enhanced Turmeric Formulation on Radiation-Induced Oral Mucositis. ORL 2022, 84, 103–113.
- Rahimi, H.R.; Nedaeinia, R.; Shamloo, S.S.; Sh, N. Novel Delivery System for Natural Products: Nano-Curcumin Formulations. Avicenna J. Phytomed. 2016, 6, 383–398.
- Tahmasebi, S.; Saeed, B.Q.; Temirgalieva, E.; Yumashev, A.V.; El-Esawi, M.A.; Navashenaq, J.G.; Valizadeh, H.; Sadeghi, A.; Aslani, S.; Yousefi, M.; et al. Nanocurcumin Improves Treg Cell Responses in Patients with Mild and Severe SARS-CoV2. Life Sci. 2021, 276, 119437.
- Delavarian, Z.; Pakfetrat, A.; Ghazi, A.; Jaafari, M.R.; Homaei Shandiz, F.; Dalirsani, Z.; Mohammadpour, A.H.; Rahimi, H.R. Oral Administration of Nanomicelle Curcumin in the Prevention of Radiotherapy-Induced Mucositis in Head and Neck Cancers. Spec. Care Dentist. 2019, 39, 166–172.
- Kia, S.J.; Basirat, M.; Saedi, H.S.; Arab, S.A. Effects of Nanomicelle Curcumin Capsules on Prevention and Treatment of Oral Mucosits in Patients under Chemotherapy with or without Head and Neck Radiotherapy: A Randomized Clinical Trial. BMC Complement. Med. Ther. 2021, 21, 1–11.
- Ramezani, V.; Ghadirian, S.; Shabani, M.; Boroumand, M.A.; Daneshvar, R.; Saghafi, F. Efficacy of Curcumin for Amelioration of Radiotherapy-Induced Oral Mucositis: A Preliminary Randomized Controlled Clinical Trial. BMC Cancer 2023, 23, 1–9.
This entry is offline, you can click here to edit this entry!
