Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, General & Internal
Sarcopenia is an age-related clinical complaint characterized by the progressive deterioration of skeletal muscle mass and strength over time. Type 2 diabetes (T2D) is associated with faster and more relevant skeletal muscle impairment. Both conditions influence each other, leading to negative consequences on glycemic control, cardiovascular risk, general health status, risk of falls, frailty, overall quality of life, and mortality.
- sarcopenia
- diabetes mellitus
- obesity
- aging
- physical exercise
1. Introduction
Sarcopenia is defined as an age-related impairment of skeletal muscle performance, resulting in progressive deterioration of mobility, increased risk of falls and fractures, impaired ability to carry out daily activities [1]. According to the European Working Group on Sarcopenia in Older People, sarcopenia may occur with one or more three specific criteria: (a) low muscle strength, (b) low muscle quantity or quality, and (c) low physical performance [2]. Sarcopenia should be suspected if one criterion is satisfied. Two criteria confirm the diagnosis, and three define “severe sarcopenia”. Similarly, according to the Third National Health and Nutrition Examination Survey, muscle mass should be assessed by bioimpedance analysis and be expressed as skeletal muscle index (skeletal muscle mass-to-body mass index × 100). Sarcopenia is defined when individual skeletal muscle index is lower than one standard deviation compared to reference values [3].
Type 2 diabetes mellitus (T2D) is a chronic multifactorial and systemic disease characterized by hyperglycemia and hyperglycemia-induced deterioration of microcirculation and macrovascular complications. The prevalence of T2D increases with age; hence, an overlap between T2D and sarcopenia is anticipated.
Sarcopenia is more prevalent in patients with chronic diseases, such as T2D, indicating that the age-related decline in skeletal muscle performance is faster than in healthy individuals [4,5]. Poor glucose control, longer diabetes evolution, and the presence of chronic diabetes-related complications also increase the risk of sarcopenia in T2D [6,7,8,9].
2. Mechanism of Diabetes-Induced Sarcopenia
Evidence suggests that insulin resistance is associated with impaired skeletal muscle glucose uptake and utilization and intracellular accumulation of triglycerides/fatty acids, both associated with sarcopenia [10]. Lipid accumulation in myocytes further reduces skeletal muscle sensitivity to insulin [11]. Insulin resistance, hyperglycemia, and T2D per se induce mitochondrial dysfunction, impaired oxidative metabolism, and energetic utilization, contributing to sarcopenia [12]. In addition, insulin resistance impairs post-prandial myofibrillar protein synthesis due to an imbalance between catabolic and anabolic stimuli at the skeletal muscle site [13].
Proinflammatory cytokines, such as interleukin 1b (IL1b) and tumor necrosis factor α (TNFα), exacerbate protein imbalance by acting as catabolic stimuli [9]. Circulating levels of these cytokines are elevated in T2D and related co-morbidities, thus contributing to background systemic inflammation [14]. Resident macrophages are known to induce and sustain inflammation in the adipose tissue, pancreatic islets, liver, and other peripheral tissues [10], with a significant contribution to the pathophysiology of T2D. As another mechanism, proinflammatory macrophages promote lipolysis, thus exacerbating skeletal muscle steatosis and insulin resistance [12]. Advanced glycation end products (AGEs) also contribute to systemic inflammation and sarcopenia. The effect is attributable to the AGE-mediated activation of scavenger receptors (RAGEs), leading to the activation of proinflammatory pathways associated with systemic inflammation (NF-kB) and oxidative stress (NADPH oxidase) [15]. Hence, background systemic inflammation fosters sarcopenia in patients with T2D and related co-morbidities.
Gut dysbiosis plays a role in chronic intestinal and systemic diseases, including T2D. Notably, the balance between Bifidobacteria and Bacteroides is crucial in maintaining a healthy intestinal barrier. Children develop the best composition of the gut microbiome when vaginally born and breastfed with maternal milk. Once solid foods are consumed daily, Bacteroides and Firmicutes become the prevalent species [16]. Subsequent changes in microbiome composition are related to genetic predisposition and diet. A hypercaloric Western diet predisposes one to T2D and cardiometabolic complications and is characterized by a significant shift in microbiome composition, expressed as a high Bacteroides to Bifidobacterial ratio. The mechanisms explaining the relation between gut dysbiosis and T2D are related to a significant change in intestinal mucosal membrane permeability that facilitates bacterial leakage and translocation from the gut lumen to the subepithelial space, eventually promoting endotoxemia, systemic inflammation, impaired insulin synthesis, and insulin resistance [17]. In addition, some specific species, such as Akkermansia muciniphila, are also less expressed in the gut of individuals with T2D, and this phenomenon is associated with impaired intestinal membrane permeability due to reduced synthesis of mucins, exacerbating bacterial leakage as mentioned above [18]. Moreover, gut dysbiosis is associated with defective synthesis of essential micronutrients, such as vitamin B12 and tryptophan, that play a crucial role in skeletal muscle homeostasis, with the latter phenomenon explaining the role of gut dysbiosis in T2D and sarcopenia [19].
Suboptimal chronic protein intake is an age-related nutritional concern. Several factors influence protein intake with advancing age, including physiological changes, such as reduced daily energy requirement, genetic predispositions to low appetite, dental issues, impaired gastric acid secretion and slow gastric emptying, pathological conditions, including physical and mental disabilities, inability to prepare or consume food, dysphagia, and environmental factors such as financial concerns or loneliness [20]. Moreover, these background conditions also affect bromatological diet composition in favor of carbohydrates (and rapidly adsorbed carbohydrates), thus increasing the risk of T2D, obesity, and sarcopenia.
Vitamin D (Vit-D) deficiency and insufficiency are frequently observed in old people. The leading causes of the age-related fall in Vit-D levels are attributable to low intake of naturally Vit-D-rich foods (e.g., meats, fish, eggs, milk, and milk-derived foods) and impaired dermal Vit-D metabolism. Vit-D deficiency is a usual finding in T2D. Vit-D deficiency contributes to impaired insulin synthesis and insulin resistance, increasing the risk of prediabetes and T2D [21]. Vit-D deficiency also contributes to sarcopenia, osteomalacia, osteoporosis, and the risk of falls and fractures [22].
Hormonal changes occur along with aging. The decline in the frequency and amplitude of growth hormone (GH) peaks and insulin-like growth factor (IGF) 1 is observed in old patients [23]. A similar imbalance is also known for testosterone in men and estrogen, progesterone, and ovarian- and adrenal-derived androgens in women [24,25]. T2D is frequently associated with male hypogonadism, with both conditions fostering sarcopenia in affected men [26]. Figure 1 depicts the pathogenesis of diabetes-related sarcopenia and the potential mechanisms to restore healthy skeletal muscle from sarcopenia.
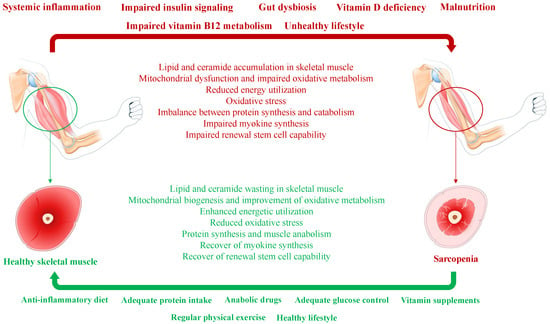
Figure 1. Simplified pathogenesis of diabetes-related sarcopenia and reverting mechanisms involved in the switch from sarcopenia to healthy skeletal muscle.
T2D is associated with insulin resistance, chronic (low-grade) systemic inflammation, unhealthy lifestyle, malnutrition, and microbiome changes that represent concurrent factors of sarcopenia (indicated in bold red above the horizontal red arrow). Concurrent factors induce a significant perturbation in the physiological functions and biochemical activities of skeletal muscle (shown in red below the horizontal red arrow). A healthy lifestyle, including diet, protein and vitamin supplementation, regular physical exercise, and anabolic supplementation when necessary (indicated in bold green below the horizontal green arrow), attenuates skeletal muscle catabolism and may revert sarcopenia to healthy skeletal muscle (shown in green above the horizontal green arrow).
3. Sarcopenic Obesity
The term sarcopenic obesity defines a chronic condition in which obesity, T2D, and sarcopenia coexist. Sarcopenic obesity, compared to obesity alone, negatively affects the quality of life and increases the risk of cardiometabolic disorders and overall mortality [27].
It has been estimated that around 30% of older Italian patients diagnosed with sarcopenia had a concomitant condition of sarcopenic obesity, and T2D increased the risk of sarcopenic obesity by 73% [28]. According to the New Mexico Aging Process Study [29], sarcopenic obesity is diagnosed when skeletal muscle mass is at least two standard deviations below the mean reference value for weight-normalized skeletal muscle mass, i.e., <7.26 kg/m2 in men and <5.45 kg/m2 in women, and body fat mass is greater than 27% in men and 38% in women. According to the Third National Health and Nutrition Examination Survey [30], sarcopenic obesity occurs when skeletal muscle mass is less than 9.12 kg/m2 in men and <6.53 kg/m2 in women and fat mass >37.16% in men and >40% in women.
The mechanisms involved in sarcopenic obesity are similar to those described for sarcopenia. Apart from insulin resistance, systemic inflammation, physical inactivity, and malnutrition, patients with sarcopenic obesity usually display marked hormonal impairment. Low circulating levels and marked impairment of liver sensitivity to GH and, consequently, low circulating levels of IGF 1 have been reported, as well as functional hypogonadism in men [31].
In addition, dysfunctional skeletal muscle to adipose tissue crosstalk is involved in the pathogenesis of sarcopenic obesity. Interleukin 6 (IL6) is secreted by several types of cells, including striate myocytes. In healthy individuals, skeletal muscle activation leads to an acute increase in circulating levels of IL6 during and hours after the conclusion of a bout of exercise. This sharp rise in IL6 is not detrimental. Instead, it is followed by an improvement in insulin sensitivity, which in turn facilitates glucose uptake and protein synthesis in skeletal muscle [32]. Conversely, chronic overexposure to IL6 due to systemic inflammation fosters T2D and sarcopenia.
4. Sarcopenia: A Determinant of Glucose Deterioration and Poor Outcomes
Sarcopenia is associated with low glucose disposal at the skeletal muscle site [33]. Skeletal muscle is responsible for around 80% of glucose uptake during experimental conditions of euglycemic hyperinsulinemic clamp [34]. Skeletal muscle serves as a sort of buffer against hyperglycemia after a glucose load, as observed in the post-prandial phase under physiological conditions [35]. Preserving skeletal muscle mass prevents the onset of prediabetes and progression to T2D [34], as healthy insulin-sensitive skeletal muscle is essential to regulate glucose disposal. First, insulin stimulates the endothelial expression of nitric oxide synthase, nitric oxide production, and peripheral vasodilation. This mechanism ensures adequate blood flow and nutrient supply to skeletal muscle. Second, insulin stimulates the Akt/PKB-mediated translocation of glucose transporters, such as GLUT4, on myocyte membranes. Therefore, insulin is essential in increasing overall glucose uptake in skeletal muscle [36]. The third mechanism is insulin-independent and involves an extracellular matrix interposing the space between microvascular vessels and myocytes. In T2D, myocyte steatosis prompts insulin resistance, oxidative stress, cell injury, necrosis, and apoptosis. All these events stimulate the recruitment and translocation of peripheral monocyte/macrophage-derived proinflammatory cells into skeletal muscles, resulting in local inflammation, accumulation of cellular debris, and fibrillar amorphous matrix. The extracellular matrix becomes a thicker tissue, hindering glucose transport from vessels to myocytes. Inflammation, insulin resistance, and impaired regulation of intramuscular blood flow significantly affect glucose disposal by skeletal muscle [37].
Myokines are a group of proteins with autocrine, paracrine, and endocrine activities, and are produced and released by myocytes, whose expression increases with healthy skeletal muscle [38,39]. These molecules control muscle metabolism and growth, and have immunoregulatory effects [40]. Myokines can be classified as positive and negative regulators of muscle growth, differentiation, and repair. Bone-morphogenic proteins and irisin are the leading positive regulators, along with follistatin, which is secreted at the liver site. Myostatin, transforming growth factor β, activins, and growth differentiation factor are the foremost negative regulators [41,42]. A negative balance between myokines affects the differentiation, proliferation, and repair of myocytes and impairs myofibrillar synthesis, leading to sarcopenia [43]. Myokines, such as IL6, IL10, IL15, irisin, myonectin, osteocorin, and secreted proteins acidic and rich in cysteine (SPARC), are also involved in the crosstalk between skeletal muscle and peripheral tissues, such as pancreatic islets, the liver, adipose tissue, and in the regulation of insulin sensitivity, glucose metabolism, metabolite utilization, and energy expenditure [44,45,46].
Physical inactivity and a sedentary lifestyle are associated with insulin resistance, poor glucose control, and metabolically related consequences, including metabolic syndrome, T2D, obesity, and cardiovascular diseases [47,48]. A sedentary lifestyle is associated with loss of mechanical stimuli, consequent impairment of skeletal muscle trophism [49], skeletal muscle loss [50], and impaired myokine secretion. All these events are involved in impaired glucose metabolism and T2D pathophysiology. Overall, sarcopenia is an independent risk factor of new-onset T2D in normal-weight older people [51], as sarcopenic compared to non-sarcopenic patients require frequently a multipharmacological approach to manage chronic diseases [52] and related outcomes [53].
This entry is adapted from the peer-reviewed paper 10.3390/nu16010063
This entry is offline, you can click here to edit this entry!
