1. Cultivation-Based Detection
With its earliest practices back in the middle of the nineteenth century, microbial cultivation has been long and widely used for isolation and identifying pathogens. Even for now, classification on microbial culture by examination of its gross morphological, macroscopic, physiology features are the benchmark for diagnosing numerous microorganisms in healthcare settings [
16]. Cultivation-based methods offer several key benefits in terms of simplicity, reliability, and the absent need for advanced equipment; however, it exhibits limitations that include a time-consuming and laborious culturing procedure, susceptibility to contamination, and a dependence on phenotypic characterization. Moreover, cultivation can be applied to a mere 2~3% of the microbial population [
17,
18]. To address these limitations, cultivation methods have been improved in recent decades, with the emergence of innovative techniques for targeted or high-throughput cultivation. These advancements involve the use of diverse and specialized growth media components, precise control of environmental conditions, the utilization of heterogeneous host cells, and the incorporation of growth-promoting factors [
19]. It is worth mentioning that the combination of microbial cultivation, typically for bacterial species, with matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF) has proven to be a highly promising strategy for identifying various microbial species. The MALDI-TOF technique is characterized by its speed, sensitivity, and cost and labor efficiency. Nonetheless, the technology’s capacity for identifying novel pathogens is confined to instances where peptide mass fingerprints from relevant reference strains are available [
20]. Given these contexts, it is concerning but perhaps not surprising that pathogen discovery continues to rely heavily upon cultivation-based methods. On 24 January 2020, China CDC isolated the first strain of the SARS-CoV-2 virus from the lower respiratory alveolar lavage fluid of a COVID-19 patient [
21]. Subsequentially, the success of viral culturing has enabled the exploration of additional valuable prospects such as electron microscope images, studies on viral transmission and viability, and experiments involving cellular and animal models.
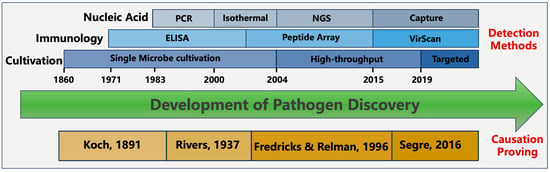
In contrast to cultivation, modernized methods of pathogen detection rely on identification of molecular signatures during infection. These molecular signatures come from either the microbial intrinsic traits or the host’s immune response to the pathogen. Depending on the types of molecular signatures, detection methods can be split into different categories, including nucleic acid-based, antigen-based, and immunology response-based detection methods (
Figure 1). Additionally, it is worth mentioning that several alternative techniques exist, such as digital pathology, advanced imaging technologies, wearable biosensors, and pathogenic-microbiome analysis [
22]. These methods have shown potential in aiding disease detection and diagnosis but are beyond the scope of this review.
Figure 1. The development of pathogen discovery.
2. Nucleic Acid-Based Detection
Nucleic acid-based methods generally involve the amplification of a specific signature of a nucleic acid sequence as its starting material is trivial to detect. When the nucleic acid is amplified with a certain detectable amount, the detection of nucleic acid can be achieved by fluorescent signaling, probe-based methods such as hybridization, direct visualization through electrophoresis, NGS sequencing, CRISPR, and others [
23].
Polymerase Chain Reaction (PCR) is the most widely used method, which involves a series of heating and cooling cycles to separate the DNA strands, bind primers to the target sequence, and amplify the DNA fragment using DNA polymerase enzyme. Reverse Transcription Polymerase Chain Reaction (RT-PCR) was to first convert the RNA template into complementary DNA (cDNA) using reverse transcriptase enzyme, and then followed by regular PCR. The gold-standard diagnosis method of COVID-19 is RT-PCR, and trillions of real time RT-PCR tests have been carried out around the globe during the COVID-19 pandemic [
24]. The use of COVID-19 qPCR testing directly enables early detection, contact tracing, identification of asymptomatic carriers, monitoring, and surveillance, mitigating cluster outbreaks and assessing the effectiveness of control measures.
In comparison to PCR-based methods, isothermal amplification technology offers efficient and specific DNA amplification under constant temperature conditions. This eliminates the need for complex equipment [
25] and reduces the amplification time [
23]. This technology also demonstrates greater tolerance towards impurities and inhibitors commonly found in field samples, ensuring high sensitivity and specificity without the necessity for purification or specialized handling. Moreover, isothermal amplification allows for amplification to be initiated using smaller amounts of target biomass but longer amplicons. These inherent advantages make isothermal amplification technology suitable for integration into portable biosensing devices, allowing improved sensitivity with on-site testing. However, isothermal amplification also encounters certain challenges like intricate design of primers, amplification that lacks specificity, high background signal, and the need for additional and costly enzymes and denaturing agents [
26]. Collectively, the user-friendly nature of isothermal amplification technology makes itself an attractive candidate for point-of-care molecular tests. In the past four years, isothermal amplification market has gained huge recognition, and it is expected to grow at a compound annual growth rate of 12.2% during the next forecast decade. Popular isothermal amplification tests include RT-RPA (Recombinase Polymerase Amplification) [
27], LAMP (Loop-Mediated Isothermal Amplification) [
28], and NEAA (Nicking enzyme-assisted amplification) [
29].
CRISPR-Cas-based detection relies on hybridization of the Cas protein with the DNA motif signatures of pathogens [
30]. The hybridization event illustrates the presence of pathogenic DNA and generates a measurable signal, which can be fluorescence, electrochemical signal, colorimetric reaction, or a visual signal on a lateral flow device. CRISPR-coupled RT-RPA or RT-LAMP are advantageous alternatives to RT-PCR due to their cost-effectiveness and faster turnaround time. Currently, the lowest limit of detection (LOD) achieved among the CRISPR methods was two RNA copies per sample [
31]. It has also been reported this test can be performed at a cost of less than USD 3.5, with a potential production scale cost as low as USD 0.7 [
32]. The average accuracy of all CRISPR methods is approximately 96.5%, and the test results can be obtained in about 50 min [
33]. Compared to RT-PCR, the CRISPR-based COVID test offers improved reliability and cost-effectiveness, making it a viable alternative. In combination with LAMP, CRISPR/cas-based assays have gained US FDA emergency use authorization during the COVID-19 pandemic.
Metagenomic Next Generation Sequencing (mNGS) is another technology that allows sequencing of DNA from various microbial species present in a sample simultaneously. The method involves extracting total DNA from the sample, followed by shotgun sequencing of DNA fragments that are assembled into contigs. These contigs can be then utilized to identify the presence of pathogens, microbial communities, and their genetic characteristics. In the past decade, mNGS has become an increasingly popular technique for diagnosing infections and characterizing microbial communities. When compared to alternative diagnostic technologies, one key advantage of mNGS is its unbiased sampling trait, allowing for the comprehensive identification of known pathogens, unexpected pathogens, and even the discovery of new organisms. The first evidence of SARS-CoV-2 was detected by metagenomics screening on unknown febrile patients from Wuhan, China, in late December 2019 before it came to public awareness. Another advantage is its ability to provide supplementary genomic information that supports evolutionary tracing, strain identification, and prediction of drug resistance. Furthermore, mNGS provide quantitative or semi-quantitative data by counting the number of sequenced reads, which is especially valuable for polymicrobial samples or cases where multiple pathogens are implicated in the disease process. It is also worth highlighting that the third generation of sequencing technology can provide high confidence and real-time surveillance during the COVID-19 pandemic, as demonstrated in numerous instances [
34,
35]. The global metagenomic sequencing market in terms of revenue was estimated to be worth USD 2.0 billion in 2023 and is poised to reach USD 4.5 billion in 2028, growing at a compound annual growth rate of 17.5% from 2023 to 2028.
A meta-analysis on the diagnostic test accuracy of mNGS indicates that sensitivity and specificity were 90% and 86% for blood, 75% and 96% for cerebrospinal fluid (CSF), and 84% and 67% for orthopedic samples, respectively [
36]. However, the implementation of mNGS in clinical diagnostic laboratories has been limited, primarily due to operational complexity, cost considerations, and insufficient sensitivity compared to agent-specific PCR assays. Several strategies have been proposed to improve the sensitivity of mNGS, including the enrichment of pathogen templates through the removal of host nucleic acids using nuclease digestion and the depletion of ribosomal RNA [
10]. Although these strategies have been helpful, none have achieved the sensitivity required for clinical applications. Aiming to enhance the sensitivity of mNGS and make it more suitable for clinical diagnostic purposes, a positive selection probe capture-based system was developed for the enrichment of targeted sequences [
37,
38,
39]. Compared to the unbiased mNGS, capture-based sequencing can increase the sensitivity up to 1000-fold, reaching the detection sensitivity which is equivalent to RT-PCR [
39]. A baited probe can be customized into different designs for specific usage. Among different laboratory developed tests, VirCapSeq has been first certified by the New York State Department of Health Clinical Laboratory Evaluation Program as viral pathogen detection for use in patient diagnosis and public health surveillance. When it comes to bacterial or fungal infections, where the species identification of pathogens can impact therapeutic decisions due to intrinsic resistance from antimicrobial resistance genes, the effect of antibiotics administration can be significantly improved through AMR detection associated with bacterial and fungal pathogens [
40]. However, it is worth noting that capture-based sequencing has limitations in detecting novel pathogens that lack nucleotide sequence similarity represented in the probe-target pools, and its sequencing results may result in biased representation of sequences, reflecting variations in gene expression patterns and primer biases during library preparation.
3. Antigen-Based Detection
Aiming to provide quick and accessible testing options, the antigen-based detection is to identify the presence of antigens from the pathogen. Indeed, most self-tests or at-home tests are based on antigen detection. The COVID-19 pandemic also marked a milestone in public health as it witnessed the widespread adoption of self-testing by individuals, primarily with the SARS-CoV-2 antigen test. By empowering individuals to take an active role in their own testing, it enhances accessibility and convenience, leading to earlier detection, isolation, and treatment of infected individuals. Self-testing also reduces the burden on healthcare systems, allowing resources to be focused on those who require critical care. The two primary antigens targeted in SARS-CoV-2 detection assays are the spike protein (S protein) and the nucleocapsid protein (N protein) [
41]. In the recent Cochrane review on various commercial SARS-CoV-2 antigen-detection rapid diagnostic tests (Ag-RDTs), it has been reported with a sensitivity of 56.2% (95% CI, 29.5% to 79.8%) and specificity of 99.5% (95% CI, 98.1% to 99.9%) [
42]. The characteristic of Ag-RDTs determines that it can be effectively used in the first week after symptoms begin to confirm the infection. It is important to note that the effectiveness of Ag-RDTs in detecting infections may vary depending on the specific test used, the quality of the product, and the timing of testing in relation to symptom onset. While Ag-RDTs offer a valuable tool for early diagnosis during the first week of symptoms, confirmatory testing with other molecular methods, such as PCR, is still recommended in cases where Ag-RDT results are negative, but clinical suspicion remains high. This combined approach helps to enhance the accuracy of diagnosis and ensure that appropriate public health measures are implemented for effective control of the infection [
43].
4. Antibody-Based Detection
Serological methods based on antibodies are used to target specific immunoglobulin isotypes (IgA, IgM, or IgG) or total antibodies in blood samples and occasionally in other body fluids like saliva. Serological diagnosis is essential when the genetic material (nucleic acid) of the infectious agent cannot be detected, at least 1 to 2 weeks after symptom onset for the host to develop immune responses. These assays offer valuable information about the humoral immune responses and the specific antigens they recognize, making them particularly useful for investigating complex chronic diseases and relevant molecular epidemiology research after outbreaks [
11]. Known as ELISA, the earliest serological assay was first developed in 1971 [
44]. Despite being considered labor-intensive and expensive, ELISA has been widely used in the clinical diagnosis and laboratory research. Regarding serological assays for SARS-CoV-2, the nucleocapsid (N) protein and the receptor-binding domain (RBD) of the S1 subunit of the S glycoprotein are the most frequently used antigens [
45]. IgA and IgM have demonstrated advantages in detecting early immune responses, but concerns have been raised regarding their shorter lifespan compared to IgG in serum and saliva [
46].
Antibody-based detection has also explored its potential in the high throughput form. One strategy is the use of a programmable microarray that consisted of a massive number of synthetic peptides. Depending on the material of the slide carrier, the array can accommodate from a range of from thousands to three million distinct linear peptides. This approach has greatly enhanced the assay sensitivity, enabling early diagnosis of diseases for Lyme disease, Acute Flaccid Myelitis, and others [
47,
48,
49,
50]. Another technique, VirScan, integrates high-throughput DNA oligo synthesis, bacteriophage display, and next-generation sequencing to achieve antibody profiling. By screening sera samples against a library of viral peptides, VirScan can identify the specific peptides recognized by antibodies through immunoprecipitation and sequencing [
51]. VirScan has been successfully used to map the immune response to the SARS-CoV-2 virus in COVID-19 patients, providing insights into cross-reactivity and disease severity factors [
52]. PepSeq is another multiplexed proteomic assay for pathogen discovery. Its protocol involves synthesizing the peptides of customizable targets of interest and linking them to cDNA tags in an in vitro and massively parallel manner. The resulting libraries enable highly multiplexed assays that utilize high-throughput sequencing to profile the binding or enzymatic specificities for targeted peptides [
53].
This entry is adapted from the peer-reviewed paper 10.3390/pathogens13010051