2. Immunosuppression during Acute and Chronic Inflammation
Regarding the time elapsed, an acute inflammation can be subdivided into two major phases with distinct differences in the mediator profile and immune cell functions. First, in response to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), pro-inflammatory cascades are induced via the activation of pattern recognition receptors (PRRs) [
17,
18]. As a result, immune cells are recruited and activated onto inflammatory sites and the maturation of dendritic cells is induced for the presentation of antigens to T cells [
19]. Typical pro-inflammatory mediators are cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor α (TNF-α), and the acute-phase proteins C-reactive protein (CRP) and serum amyloid A (SAA) [
4,
5,
20,
21,
22]. These activities are directed to deactivate pathogens, eliminate virus-infected or transformed cells, remove any damaged cell material, and create better conditions for ongoing immune reactions. This very common concept of pattern recognition is valid for the initiation of novel inflammatory events in a broad range of diseases, including infections, rheumatoid arthritis, atherosclerosis, Alzheimer’s disease, and many others, as well as tumors.
During inflammatory response, different host-derived cytotoxic agents are released or generated, which are derived from activated immune and undergoing tissue cells, and in tumors also from tumor cells. These agents generally execute a dual role in living tissues. They are an essential part in many physiological functions, especially in immune cell-triggered processes. Cytotoxic agents are mandatory for combatting microbes, to restore tissue homeostasis after any threat, and to ensure normal functioning of the physiological processes in the organism [
4]. Otherwise, they can damage unperturbed tissues and contribute to the initiation of novel inflammatory cascades. According to their mode of action, cytotoxic agents can be differentiated into oxidant-based agents (reactive species, oxidized heme proteins, free heme, transition metal ions) and protease-based agents (serine proteases, matrix metalloproteases, pro-inflammatory peptides) [
16]. In healthy tissues, cytotoxic agents are inactivated by already existing antagonizing principles.
In the second phase of an acute inflammation, the termination or resolution phase, destroyed cells and tissues are replaced by novel synthesized material via the induction of proliferative processes. The pro-inflammatory activities of immune cells are downregulated, and finally the former homeostasis is restored. The typical transiently acting cytokines of this phase are transforming growth factor β (TGF-β) and IL-10 [
23,
24,
25]. In addition, growth factors [
26,
27] and lipid mediators, such as lipoxins and resolvins, are involved in the resolution of inflammation [
28,
29]. Cell signaling is characterized by the induction of the STAT3 pathway. Macrophages are polarized from the M1 type to M2 type [
30,
31]. The accumulation of myeloid-derived suppressor cells (MDSCs) contributes to the development of a transient immunosuppression in inflamed areas [
32,
33]. MDSCs are immature immune cells that promote immunosuppressive effects on lymphocytes, natural killer cells, macrophages, and dendritic cells [
34]. In general, the aforementioned mechanisms impede the hyperactivation of pro-inflammatory cells and prevent any excessive tissue damage by host-derived cytotoxic agents during the resolution of inflammation. After the resolution, the number of immune cells and mediators is reduced to a level typical of healthy tissues [
35]. Cytotoxic agents and their antagonizing counterparts are also involved in the resolution of inflammation, especially in the processes of matrix remodeling.
Under chronic inflammatory conditions, inflammation is only insufficiently terminated as pro-inflammatory cascades are activated again and again due to the incomplete inactivation of host-derived cytotoxic agents and the continuing release of DAMPs and antigens. At chronic inflammatory sites, pro-inflammatory mediators, the recruitment of immune cells, and tissue damage co-exist with a plethora of immunosuppressive mechanisms, such as the prevalence of MDSCs, the presence of inflammation-resolving and proliferative factors, as well as the depletion of essential metabolites for appropriate immune defense [
16]. The resulting long-lasting immunosuppression can seriously affect the health status and enhance susceptibility to chronic infections and comorbidities [
36,
37]. For example, in elderly persons, persistent inflammation is closely associated with immune dysfunction. This condition is known as inflammaging [
38], which is regarded as a risk factor for life-threatening diseases and adverse health outcomes [
39,
40]. In general, pro-inflammatory mechanisms and immunosuppressive conditions are closely associated with each other in many chronic diseases, including cancer.
In chronic inflammations, serious health problems can arise from the excessive release or generation of cytotoxic agents and from the decline or exhaustion of protective mechanisms. In consequence, the following release of novel DAMPs, antigens, and cytotoxic agents promotes ongoing inflammatory events and prevents the termination of inflammatory cascades. The worst case is a very low capacity of antagonizing principles or their exhaustion with the consequence of septic complications and organ failure [
16]. This concept of the incomplete inactivation of cytotoxic agents by protective principles explains under which conditions an inflammation becomes persistent. It also provides the basis for a better understanding of the underlying molecular mechanisms for a wide range of chronic inflammatory diseases, including cancer.
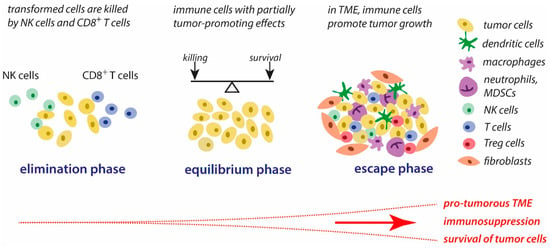
In the description of the molecular processes during tumorigenesis, numerous cytotoxic agents derived from tumor cells, immune cells, and other tumor-associated cells are more expressed and exhibit higher activities compared to healthy tissue areas. In general, these agents are able to damage transformed cells with the subsequent elimination of these cells by the immune system. This is mostly observed during the early phases of tumorigenesis. However, in response to stress and the increasing accumulation of cytotoxic agents, numerous antagonizing principles are markedly upregulated in advanced tumors and promote the survival of tumor cells and protection against stress induced by chemo- and radiotherapy.
3. Key Elements of Immunosuppression in Tumors
Tumor progression and chronic inflammatory processes are highly linked to each other [
1,
2,
3,
6]. In the creation of an immunosuppressive milieu, both tumor cells and the TME contribute multiple mechanisms to the manipulation of the host’s immune answer and to tissue remodeling in tumors. An overview of the major mechanisms of tumor cell-mediated processes in the TME and immunosuppression is given in
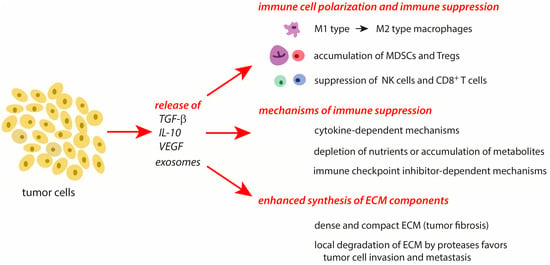
Figure 2.
Figure 2. Major effects of tumor cells on the surrounding cells in the TME. The effects are mediated by the release of mediators from tumors cells and special conditions typical of tumors. Further explanations are given in the text. Abbreviations: IL-10—interleukin 10, MDSCs—myeloid-derived suppressor cells, NK cells—natural killer cells, TGF-β—transforming growth factor-β, Tregs—regulatory T cells, VEGF—vascular endothelial growth factor.
During tumor progression, the principal processes involved in immunosuppression and tissue remodeling during the resolution phase of an acute inflammation are also active. Whereas these mechanisms are only transiently expressed in a subsiding inflammation, they act permanently and more intensively in tumors. In cancer cells, several protective systems are highly upregulated in response to a stress-mediated increase of cytotoxic agents. Further, these cells actively secrete inflammation-resolving molecules (TGF-β, IL-10) and exosomes that affect the conditions and properties of immune cells in the TME [
41]. Importantly, natural killer cells (NK) and cytotoxic T cells, which are able to deactivate tumor cells, are suppressed in their activity by the release of TGF-β [
42,
43,
44]. In the TME, tumor-associated macrophages (TAMs) are triggered from the pro-inflammatory M1 type to the immune-resolving M2 type [
45]. Regulatory T cells (Treg) and various types of myeloid-derived suppressor cells (MDSCs) accumulate in the TME during tumor progression. These cell types suppress the activation of other immune cells and contribute to the survival of tumors [
3,
13,
32,
35,
41,
46,
47,
48]. With their cargo, cancer cell-derived exosomes are able to modulate different processes of tumor progression such as angiogenesis, metastasis, and survival [
41].
MDSCs are formed as all other myeloid cells from precursors in the bone marrow. Under inflammatory conditions, the need for novel immune cells increases and these cells undergo a forced formation process, known as emergency myelopoiesis, leading to immature properties. In most kinds of cancers, the accumulation of granulocytic MDSCs (G-MDSCs) dominates over monocytic MDSCs (M-MDSCs) [
49]. In glioma, M-MDSCs are more expressed than G-MDSCs [
49]. In humans, a third, less pronounced type of MDSCs, early-stage MDSCs (e-MDSCs), has been described [
50,
51]. The MDSC subtypes are usually identified by their morphological properties, density, and surface markers [
49].
In the TME, immunosuppressive conditions can be differentiated by their underlying mechanisms as enzyme-dependent, cytokine-dependent, and immune checkpoint-dependent [
14]. In addition, the expression of neoantigens and antigen-presenting molecules like MHC-1 are decreased in cancer cells [
52,
53].
Enzyme-dependent immunosuppressive pathways are characterized by a depletion of essential metabolites, such as ATP and tryptophan, or by an enhanced formation of metabolites like adenosine, citrulline, kynurenine, and prostaglandin E2 [
14,
54,
55]. Several proteins (for example ectonucleotidases CD39 and CD73 [
56] and indoleamine 2,3-dioxygenase [
57]), which are involved in these pathways, are highly upregulated in tumors.
Cytokine-dependent immunosuppression can be induced by the activation of the IL-6/STAT-3 pathway that inhibits DC maturation and activates effector T cells [
58,
59]. Vascular endothelial growth factor A (VEGF-A) is immunosuppressive by the inhibition of the DC functions and maturation, by the infiltration of immunosuppressive cells (Treg, MDSC, and TAMs) into tumors, by reducing cytotoxic CD8
+ T cell infiltration into tumors, and by the expression of the factors involved in the exhaustion of these cells [
60,
61,
62]. Cytokines of the IL-10 and TGF-β pathways activate Treg and promote immunosuppression [
63].
Several immune checkpoints are known to be involved in immunosuppressive activities. The inactivation of T cells is possible via the binding of a specific ligand to the inhibitory T cell receptor programmed cell death protein 1 (PD-1) [
14]. This ligand known as PD-L1 or CD274 is expressed on the surface of a tumor and various other cells. The overexpression of PD-L1 on tumor cells is associated with a poor prognosis and the evasion of T cell recognition of cancers [
64]. The PD-1/PD-L1 axis represents an important element in the generation of an immunosuppressive TME [
14]. Other inhibitory immune checkpoint molecules on T cells are cytotoxic T lymphocyte-associated protein 4 (also known as CD 152), LAG3, TIM-3, and the tyrosine-based inhibitory domain (TIGIT) [
65,
66,
67]. Cancer cells can express TIGIT ligands, such as CD155, and overcome cancer immunity.
In growing and metastasizing tumors, the balance between tumor-promoting and tumor-destroying factors is shifted towards the first types of factors. Although, many features of tumor-induced immunosuppression are known, this knowledge is incomplete concerning the interrelations between them and the consequences for further tumor processing. Numerous anti-tumor therapies, such as targeted therapies and chemotherapies, attempt to overcome immunosuppression in tumors. The application of immune checkpoint inhibitors provides a novel tool for the development of combined therapy approaches [
14].
4. Poor Quality of the Tumor Vasculature
Tumorigenesis starts with the formation and uncontrolled growth of transformed cells. In developing tumors, the supply with dioxygen and nutrients is diminished due to the poor quality of the tumor vasculature, longer diffusion paths, insufficient lymphatic drainage, fluctuations in interstitial pressure, and intermittent vascular collapse in contrast to healthy tissues [
68,
69].
In solid tumors, the growth factor VEGF is overexpressed, whereby hypoxia promotes this expression [
70,
71]. This growth factor is responsible for the poor quality of the tumor vasculature with irregular, leaky, and immature vessels [
72,
73]. In addition, the VEGF is a key angiogenic factor in tumors and is involved in tumor progression and metastasis [
73,
74].
5. Direct Effects of Hypoxia in Tumor Cells
In tumors, hypoxia [
75,
76,
77] provokes significant metabolic alterations in tumor cells, such as the activation and stabilization of hypoxia-inducible factor 1α (HIF-1α) [
78], upregulation of glycolysis [
79], downregulation of oxidative phosphorylation in the mitochondria [
79], and enhanced formation of reactive species by dysfunctional mitochondria [
80]. In addition, lactate accumulates [
81,
82] and induces numerous lactate-driven effects [
81,
83,
84].
Cytosolic HIF-1α is a master regulator of glycolysis in many cells. This usually short-lived factor is controlled by prolyl hydroxylase that tags HIF-1α for proteasome degradation [
85,
86]. Hypoxia, stress-induced oxidation of Fe
2+ in propyl hydroxylase, and an ascorbate deficiency prevent HIF-1α degradation [
78]. As a result, HIF-1α upregulates several enzymes promoting glycolysis and downregulates pyruvate dehydrogenase that supplies acetyl-coenzyme A (Ac-CoA) for the citrate cycle [
79]. Unlike HIF-1α, which is a ubiquitous protein, HIF-2α is predominantly expressed in highly vascularized tissues [
87]. HIF-2α is active during prolonged hypoxia, replaces HIF-1α in a spatiotemporal manner [
75,
88], and is involved in controlling oxidative stress, the cell cycle, blood vessel remodeling, and RNA transport [
89].
In healthy cells, lipogenesis is mostly induced by pyruvate-derived Ac-CoA. To ensure lipogenesis in hypoxic cancer cells, decreased pyruvate is replaced in the TCA cycle by glutamine, supporting the conversion of α-ketoglutarate into citrate and Ac-CoA [
90]. In cancer cell growth, glutamine is an important carbon and nitrogen source for lipid, amino acid, and nucleotide synthesis [
91].
In tumorigenesis, increased lactate production by tumors cells reprograms macrophages, T cells, and other immune cells in a way that they are immunosuppressive and anti-inflammatory [
81,
82,
83,
84]. Lactate also promotes angiogenesis and tumor progression [
83,
92], supporting the hyaluronan release from the adjacent fibroblasts. This hyaluronan cover protects additional tumor tissue from immune attacks [
93].
6. Stress-Related Responses in Tumor Cells
In tumor cells, enhanced intracellular levels of reactive species, which result mainly from dysfunctional mitochondria, activate the transcription factor nuclear factor-erythroid 2-related factor 2 (Nrf2) that is low expressed under normal physiological conditions [
94,
95]. In cancer cells, Nrf2 promotes the syntheses of numerous enzymes involved in antioxidative defense and contributes to a resistance against chemo- and radiotherapy.
Prolonged hypoxia also mediates the processes of autophagy [
96,
97]. In the early stages of tumorigenesis, autophagy is directed to inhibit tumor growth [
98,
99,
100]. Otherwise, in late-stage cancers, autophagy is known to stabilize cancer cells by maintaining the integrity of the mitochondria, reducing DNA damage, and increasing the resistance against stress. Under hypoxic conditions and a low supply of nutrients, autophagy can provide energy resources for the survival of cancer cells and resistance against chemotherapy [
101]. With these mechanisms, autophagy contributes to tumor development [
102,
103,
104] and facilitates metastasis [
105,
106,
107,
108].
Under stress situations, the percentage of misfolded proteins increases in the endoplasmic reticulum (ER) as a result of the action of reactive species and electrophiles. Misfolded proteins are subjected to ubiquitinylation with subsequent proteasomal degradation or autophagy. Three major stress sensors of an unfolded protein response (UPR) are activated by misfolded proteins in a wide range of tumor cells [
109]. The overexpression of chaperones or mutations in the UPR pathways are used by tumor cells to antagonize ER stress [
110,
111,
112,
113]. A hyperactive UPR promotes tumorigenesis in advanced cancers [
114].
To maintain a reducing environment in the cytosol of tumor cells, the enhanced uptake of cystine/cysteine by tumors and the conversion into glutathione are mandatory to protect cells from stress-mediated cell death [
115]. In tumors, highly increased extracellular cysteine levels are observed, whereas cysteine is slightly enhanced within tumor cells [
116]. Moreover, cysteine is mainly transported into tumor cells by the cystine/glutamate antiporter solute carrier family 7 member 11 (SLC7A11), which is widely expressed in human cancers [
117,
118]. In tumor cells, the conversion of cysteine into hydrogen sulfide activates the Nrf2-mediated gene expression of antioxidative proteins [
119,
120]. Hydrogen sulfide also accelerates the cell cycle in tumor cells [
121].
In addition, a close link exists between the upregulation of reductive glutamine metabolism, also known as reductive carboxylation, and the transport of NADPH from cytosol into the mitochondria to upregulate mitochondrial GSH and protect against reactive species [
122]. These pathways are important for anchorage-independent growth in cancer cells.
7. Intratumoral Hemorrhages
The rupture of blood vessels results in intratumoral hemorrhages, which are typical of many tumors [
123,
124]. The conditions in hemorrhages promote the formation of cytotoxic free heme and in turn the transcriptional processes in tumor cells via the binding of free heme to guanine-rich DNA and RNA structures (known as G4 elements) [
125,
126]. Free heme controls the expression of key target genes, including telomeres and oncogenes (such as
c-Myc), and favors malignant transformation [
127,
128].
8. Reversed pH Gradient in Cancer Cells
In cancer cells, the cytosolic pH is enhanced to 7.3–7.6 (versus about 7.2 in normal cells), whereas the extracellular pH is decreased to 6.8–7.0 (versus about 7.4 in normal cells) [
129,
130]. In the TME, the milieu is more acidic by about 0.3–0.7 pH units in comparison to the cytosolic pH of tumors cells [
131]. Thus, the pH gradient across the plasma membrane of cancer cells is reversed in contrast to healthy cells. These deviations are mediated by hypoxia and lactate effects and accompanied by an increased expression and activity of ion transporters in the plasma membrane and intracellular pH regulators [
132].
In many cell types, enhanced intracellular pH values by ~0.3–0.4 units are found in proliferating cells at the end of the S phase [
133,
134] and in migrating cells [
135,
136]. The reverse pH gradient in cancer cells promotes cell proliferation, survival, migration, and metastasis [
129,
130,
137,
138].
9. Matrix Remodeling in the TME
In the TME, the extracellular matrix (ECM) acts as a barrier around tumor cells against cytotoxic immune cells. Under hypoxic conditions, the stiffness of the matrix components, especially of collagen fibrils, increases due to more intense cross-linking reactions between matrix polymers [
139,
140]. These alterations are mainly caused by cancer-associated fibroblasts [
141]. Several enzymes involved in matrix remodeling and cross-linking reactions are upregulated in tumors, namely lysyl oxidase, lysyl oxidase-like proteins, collagen prolyl 4-hydroxylase, and WINT1-inducible signaling pathway protein 1 (WISP1) [
142,
143,
144,
145].
Tumor fibrosis is closely associated with an overexpression of these enzymes [
146]. As a result, ECM fibers, most of all collagens I and III, are converted into dense, linearized, and cross-linked fiber bundles with altered mechanical properties [
147,
148,
149,
150]. The enhanced stiffness of the ECM components favors the migration of single tumor cells, promotes angiogenesis, and dampens anti-tumor activities [
151]. The elevated degree of tumor fibrosis is associated with a poor prognosis in many types of cancer [
151,
152,
153].
In the TME, the deposition of the matrix components is important for angiogenesis, proliferation, tumor cell invasion, and metastasis. Several matrix metalloproteases such as MMP-2, -3, -9, and -14 are upregulated in malignant tumors [
154,
155]. An enhanced expression of heparinase also favors angiogenesis [
156,
157,
158].
The enhanced proliferation of tumor cells is also supported by increased hyaluronan production [
159,
160,
161]. Under hypoxic conditions, the production of hyaluronan is markedly increased by tumor cells [
162]. M2-type macrophages accumulate preferentially in hyaluronan-rich areas of the TME [
163]. In addition, hyaluronan favors the phenotype change in monocytes and macrophages to the M2-type [
164].