Chronic kidney disease (CKD) poses a global health challenge, engendering various physiological and metabolic shifts that significantly impact health and escalate the susceptibility to severe illnesses. It is impacting populations worldwide causing health complications and increasing the risk of serious illnesses, with high mortality rates. CKD is associated with different complex deleterious changes in a patient’s physiology and metabolic activity. They include deteriorating function and/or subsequent kidney failure, uremia, irregularities in metabolism of amino acid, lipids, minerals, and homocysteine (leads to malnutrition, anemia, vitamin deficiency, dementia, stroke and heart diseases), metabolic acidosis, insulin resistance, inflammatory and oxidative stress, dysfunction of skeletal muscle and many more. Further, other diseases or disease-causing factors (diabetes and hypertension) which coexist within CKD are associated with deteriorating the health and mortality.
- chronic kidney disease
- cellular growth homeostasis
- inflammation
- insulin resistance
- dietary interventions
1. Carbohydrate Metabolism and Its Impairment in CKD Patients
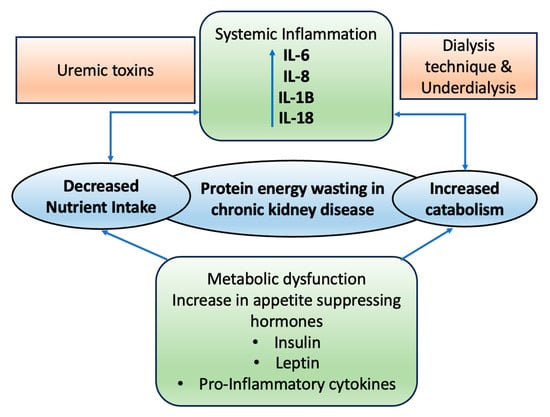
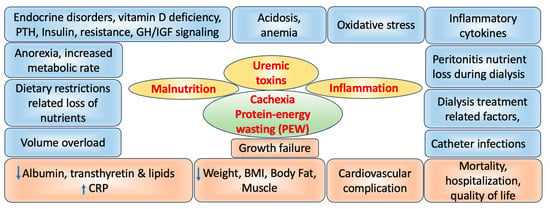
2. Role of CKD on Wasting and Malnutrition
3. Association of CKD Inflammation, Oxidative Stress and Cardiovascular Diseases
4. Effect of CKD on Functioning
4.1. Cognitive Dysfunction
4.2. On Emotional Functioning
4.3. On Taste Perception
This entry is adapted from the peer-reviewed paper 10.3390/life14010013
References
- Maughan, R.; McCommis, K.S.; Finck, B.N. Carbohydrate metabolism. Surgery 2009, 27, 6–10.
- McCommis, K.S.; Finck, B.N. Mitochondrial pyruvate transport: A historical perspective and future research directions. Biochem. J. 2015, 466, 443–454.
- Exton, J.H.; Park, C.R. Control of gluconeogenesis in liver: I. General features of gluconeogenesis in the perfused livers of rats. J. Biol. Chem. 1967, 242, 2622–2636.
- Vallon, V. Glucose transporters in the kidney in health and disease. Pflügers Archiv Eur. J. Physiol. 2020, 472, 1345–1370.
- Thomas, R.; Kanso, A.; Sedor, J.R. Chronic kidney disease and its complications. Prim. Care 2008, 35, 329–344.
- Yuen, D.A.; Stead, B.E.; Zhang, Y.; White, K.E.; Kabir, M.G.; Thai, K.; Advani, S.L.; Connelly, K.A.; Takano, T.; Zhu, L.; et al. eNOS deficiency predisposes podocytes to injury in diabetes. J. Am. Soc. Nephrol. 2012, 23, 1810–1823.
- Basturk, T.; Unsal, A. What is the frequency of carbohydrate metabolism disorder in CKD? J. Ren. Care 2012, 38, 15–21.
- Snyder, S.; Pendergraph, B. Detection and evaluation of chronic kidney disease. Am. Fam. Physician 2005, 72, 1723–1732.
- Stevens, P.E.; O’Donoghue, D.J.; De Lusignan, S.; Van Vlymen, J.; Klebe, B.; Middleton, R.; Hague, N.; New, J.; Farmer, C.K. Chronic kidney disease management in the United Kingdom: NE-OERICA project results. Kidney Int. 2007, 72, 92–99.
- Pupim, L.B.; Cuppari, L.; Ikizler, T.A. Nutrition and metabolism in kidney disease. Semin. Nephrol. 2006, 26, 134–157.
- Brem, A.S.; Lambert, C.; Hill, C.; Kitsen, J.; Shemin, D.G. Prevalence of protein malnutrition in children maintained on peritoneal dialysis. Pediatr. Nephrol. 2002, 17, 527–530.
- Sozeri, B.; Mir, S.; Kara, O.D.; Dincel, N. Growth impairment and nutritional status in children with chronic kidney disease. Iran J. Pediatr. 2011, 21, 271–277.
- Apostolou, A.; Printza, N.; Karagiozoglou-Lampoudi, T.; Dotis, J.; Papachristou, F. Nutrition assessment of children with advanced stages of chronic kidney disease-A single center study. Hippokratia 2014, 18, 212–216.
- Dai, L.; Mukai, H.; Lindholm, B.; Heimbürger, O.; Barany, P.; Stenvinkel, P.; Qureshi, A.R. Clinical global assessment of nutritional status as predictor of mortality in chronic kidney disease patients. PLoS ONE 2017, 12, e0186659.
- Mak, R.H.; Ikizler, A.T.; Kovesdy, C.P.; Raj, D.S.; Stenvinkel, P.; Kalantar-Zadeh, K. Wasting in chronic kidney disease. J. Cachexia Sarcopenia Muscle 2011, 2, 9–25.
- Mak, R.H.; Cheung, W.W.; Zhan, J.Y.; Shen, Q.; Foster, B.J. Cachexia and protein-energy wasting in children with chronic kidney disease. Pediatr. Nephrol. 2012, 27, 173–181.
- Evans, W.J.; Morley, J.E.; Argiles, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799.
- Mitch, W.E. Malnutrition: A frequent misdiagnosis for hemodialysis patients. J. Clin. Investig. 2002, 110, 437–439.
- Mitch, W.E. Insights into the abnormalities of chronic renal disease attributed to malnutrition. J. Am. Soc. Nephrol. 2002, 13 (Suppl. S1), S22–S27.
- Kalantar-Zadeh, K.; Ikizler, T.A.; Block, G.; Avram, M.M.; Kopple, J.D. Malnutrition-inflammation complex syndrome in dialysis patients: Causes and consequences. Am. J. Kidney Dis. 2003, 42, 864–881.
- Mak, R.H.; Cheung, W.; Cone, R.D.; Marks, D.L. Leptin and inflammation-associated cachexia in chronic kidney disease. Kidney Int. 2006, 69, 794–797.
- Ingulli, E.G.; Mak, R.H. Growth in children with chronic kidney disease: Role of nutrition, growth hormone, dialysis, and steroids. Curr. Opin. Pediatr. 2014, 26, 187–192.
- Mak, R.H. Cachexia in children with chronic kidney disease: Challenges in diagnosis and treatment. Curr. Opin. Support Palliat. Care 2016, 10, 293–297.
- Kovesdy, C.P.; Kopple, J.D.; Kalantar-Zadeh, K. Management of protein-energy wasting in non-dialysis-dependent chronic kidney disease: Reconciling low protein intake with nutritional therapy. Am. J. Clin. Nutr. 2013, 97, 1163–1177.
- Kopple, J.D.; Berg, R.; Houser, H.; Steinman, T.I.; Teschan, P. Nutritional status of patients with different levels of chronic renal insufficiency. Modification of diet in renal disease (MDRD) study group. Kidney Int. Suppl. 1989, 27, S184–S194.
- Hollinger, D.; Maroni, B.J.; Merrill, D.; Scherch, L.K.; Schulman, G.; Wang, S.R.; Zimmer, G.S. Relationship between nutritional status and the glomerular filtration rate: Results from the MDRD study. Kidney Int. 2000, 57, 1688–1703.
- Costelli, P.; Reffo, P.; Penna, F.; Autelli, R.; Bonelli, G.; Baccino, F.M. Ca-dependent proteolysis in muscle wasting. Int. J. Biochem. Cell Biol. 2005, 37, 2134–2146.
- Podkowińska, A.; Formanowicz, D. Chronic kidney disease as oxidative stress- and inflammatory-mediated cardiovascular disease. Antioxidants 2020, 9, 752.
- Poulianiti, K.P.; Kaltsatou, A.; Mitrou, G.I.; Jamurtas, A.Z.; Koutedakis, Y.; Maridaki, M.; Stefanidis, I.; Sakkas, G.K.; Karatzaferi, C. Systemic redox imbalance in chronic kidney disease. Oxid. Med. Cell. Longev. 2016, 2016, 8598253.
- Ling, X.C.; Kuo, K. Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018, 4, 53.
- Descamps-Latscha, B.; Drüeke, T.; Witko-Sarsat, V. Dialysis-induced oxidative stress: Biological aspects, clinical consequences, and therapy. Semin. Dial. 2001, 14, 193–199.
- Wu, C.C.; Chen, J.S.; Wu, W.M.; Liao, T.N.; Chu, P.; Lin, S.H.; Chuang, C.H.; Lin, Y.F. Myeloperoxidase serves as a marker of oxidative stress during single haemodialysis session using two different biocompatible dialysis membranes. Nephrol. Dial. Transplant. 2005, 20, 1134–1139.
- Miranda-Díaz, A.G.; Pazarín-Villaseñor, L.; Yanowsky-Escatell, F.G.; Andrade-Sierra, J. Oxidative stress in diabetic nephropathy with early chronic kidney disease. J. Diabetes Res. 2016, 2016, 7047238.
- Mahmoodpoor, F.; Saadat, Y.R.; Barzegari, A.; Ardalan, M.; Vahed, S.Z. The impact of gut microbiota on kidney function and pathogenesis. Biomed. Pharmacother. 2017, 93, 412–419.
- San, A.; Fahim, M.; Campbell, K.; Hawley, C.M.; Johnson, D.W. The role of oxidative stress and systemic inflammation in kidney disease and its associated cardiovascular risk. Nov. Prospect. Oxidative Nitrosative Stress 2018, 8, 3.
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive oxygen species in macrophages: Sources and targets. Front. Immunol. 2021, 12, 734229.
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression and Outcome. J. Immunol. Res. 2018, 2018, 2180373.
- Stenvinkel, P.; Heimburger, O.; Paultre, F.; Diczfalusy, U.; Wang, T.; Berglund, L.; Jogestrand, T. Strong association between malnutrition, inflammation and atherosclerosis in chronic renal failure. Kidney Int. 1999, 55, 1899–1911.
- Shlipak, M.G.; Fried, L.F.; Crump, C.; Bleyer, A.J.; Manolio, T.A.; Tracy, R.P.; Furberg, C.D.; Psaty, B.M. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 2002, 107, 87–92.
- Gupta, J.; Mitra, N.; Kanetsky, P.A.; Devaney, J.; Wing, M.R.; Reilly, M.; Shah, V.O.; Balakrishnan, V.S.; Guzman, N.J.; Girndt, M.; et al. Association between albuminuria, kidney function and inflammatory biomarker profile in CKD in CRIC. Clin. J. Am. Soc. Nephrol. 2012, 7, 1938–1946.
- Clapp, B.R.; Hingorani, A.D.; Kharbanda, R.K.; Mohamed-Ali, V.; Stephens, J.W.; Vallance, P.; MacAllister, R.J. Inflammation-induced endothelial dysfunction involves reduced nitric oxide bioavailability and increased oxidant stress. Cardiovasc. Res. 2004, 64, 172–178.
- Shoelson, S.E.; Lee, J.; Goldfine, A.B. Inflammation and insulin resistance. J. Clin. Investig. 2006, 116, 1793–1801.
- Navarro-Gonzalez, J.F.; Mora-Fernandez, C.; Muros de Fuentes, M.; García-Pérez, J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat. Rev. Nephrol. 2011, 7, 327–340.
- Akchurin, O.M.; Kaskel, F. Update on inflammation in chronic kidney disease. Blood Purif. 2015, 39, 84–92.
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931.
- Uchimura, T.; Nakano, K.; Hashiguchi, T.; Iwamoto, H.; Miura, K.; Yoshimura, Y.; Hanyu, N.; Hirata, K.; Imakuma, M.; Motomiya, Y.; et al. Elevation of N-(carboxymethyl) valine residue in hemoglobin of diabetic patients: Its role in the development of diabetic nephropathy. Diabetes Care 2001, 24, 891–896.
- Santhanam, A.V.; d’Uscio, L.V.; Katusic, Z.S. Erythropoietin increases bioavailability of tetrahydrobiopterin and protects cerebral microvasculature against oxidative stress induced by eNOS uncoupling. J. Neurochem. 2014, 131, 521–529.
- Schillaci, G.; Reboldi, G.; Verdecchia, P. High-normal serum creatinine concentration is a predictor of cardiovascular risk in essential hypertension. Arch. Intern. Med. 2001, 161, 886–891.
- Hailpern, S.M.; Melamed, M.L.; Cohen, H.W.; Hostetter, T.H. Moderate chronic kidney disease and cognitive function in adults 20 to 59 years of age: Third National Health and Nutrition Examination Survey (NHANES III). J. Am. Soc. Nephrol. 2007, 18, 2205–2213.
- Yaffe, K.; Ackerson, L.; Tamura, M.K.; Le Blanc, P.; Kusek, J.W.; Sehgal, A.R.; Cohen, D.; Anderson, C.; Appel, L.; DeSalvo, K.; et al. Chronic Renal Insufficiency Cohort Investigators. Chronic kidney disease and cognitive function in older adults: Findings from the Chronic Renal Insufficiency Cohort Cognitive Study. J. Am. Geriatr. Soc. 2010, 58, 338–345.
- Kurella, M.; Mapes, D.L.; Port, F.K.; Chertow, G.M. Correlates and outcomes of dementia among dialysis patients: The Dialysis Outcomes and Practice Patterns Study. Nephrol. Dial. Transplant. 2006, 21, 2543–2548.
- Griva, K.; Stygall, J.; Hankins, M.; Davenport, A.; Harrison, M.; Newman, S.P. Cognitive impairment and 7-year mortality in dialysis patients. Am. J. Kidney Dis. 2010, 56, 693–703.
- Kurella, M.C.; Glenn, M.; Luan, J.; Yaffe, K. Cognitive impairment in chronic kidney disease. J. Am. Geriatr. Soc. 2004, 52, 1863–1869.
- Murray, A.M.; Knopman, D.S. Cognitive impairment in CKD: No longer an occult burden. Am. J. Kidney Dis. 2010, 56, 615–618.
- Murray, A.M.; Tupper, D.E.; Knopman, D.S.; Gilbertson, D.T.; Pederson, S.L.; Li, S.; Smith, G.E.; Hochhalter, A.K.; Collins, A.J.; Kane, R.L. Cognitive impairment in hemodialysis patients is common. Neurology 2006, 67, 216–223.
- Weiner, D.E.; Tabatabai, S.; Tighiouart, H.; Elsayed, E.; Bansal, N.; Griffith, J.; Salem, D.N.; Levey, A.S.; Sarnak, M.J. Cardiovascular outcomes and all-cause mortality: Exploring the interaction between CKD and cardiovascular disease. Am. J. Kidney Dis. 2006, 48, 392–401.
- Matta, S.M.; Moreira, J.M.; Kummer, A.M.; Barbosa, I.G.; Teixeira, A.L.; Silva, A.C.S. Cognitive alterations in chronic kidney disease: An update. J. Bras. Nefrol. 2014, 36, 241–245.
- Drew, D.A.; Bhadelia, R.; Tighiouart, H.; Bovak, V.; Scott, T.M.; Lou, K.V.; Shaffi, K.; Weiner, D.E.; Sarnak, M.J. Anatomic brain disease in hemodialysis patients: A cross-sectional study. Am. J. Kidney Dis. 2013, 61, 271–278.
- Pringle, E.; Phillips, C.; Thijs, L.; Davidson, C.; Staessen, J.A.; de Leeuw, P.W.; Jaaskivi, M.; Nachev, C.; Parati, G.; T O’Brien, E.; et al. Systolic blood pressure variability as a risk factor for stroke and cardiovascular mortality in the elderly hypertensive population. J. Hypertens. 2003, 21, 2251–2257.
- Flythe, J.E.; Inrig, J.K.; Shafi, T.; Chang, T.I.; Cape, K.; Dinesh, K.; Kunaparaju, S.; Brunelli, S.M. Association of intradialytic blood pressure variability with increased all-cause and cardiovascular mortality in patients treated with long-term hemodialysis. Am. J. Kidney Dis. 2013, 61, 966–974.
- Chou, M.C.; Hsieh, T.J.; Lin, Y.L.; Hsieh, Y.T.; Li, W.Z.; Chang, J.M.; Ko, C.H.; Kao, E.F.; Jaw, T.S.; Liu, G.C. Widespread white matter alterations in patients with end-stage renal disease: A voxelwise diffusion tensor imaging study. Am. J. Neuroradiol. 2013, 34, 1945–1951.
- Kimmel, P.L. Depression in patients with chronic renal disease: What we know and what we need to know. J. Psychosom. Res. 2002, 53, 951–956.
- Davison, S.N.; Jhangri, G.S. Impact of pain and symptom burden on the health-related quality of life of hemodialysis patients. J. Pain Symptom Manag. 2010, 39, 477–485.
- Palmer, S.; Vecchio, M.; Craig, J.C.; Tonelli, M.; Johnson, D.W.; Nicolucci, A.; Pellegrini, F.; Saglimbene, V.; Logroscino, G.; Fishbane, S.; et al. Prevalence of depression in chronic kidney disease: Systematic review and meta-analysis of observational studies. Kidney Int. 2013, 84, 179–191.
- Hedayati, S.S.; Minhajuddin, A.T.; Afshar, M.; Toto, R.D.; Trivedi, M.H.; Rush, A.J. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA 2010, 303, 1946–1953.
- Cukor, D.; Fruchter, Y.; Ver Halen, N.; Naidoo, S.; Patel, A.; Saggi, S.J. A preliminary investigation of depression and kidney functioning in patients with chronic kidney disease. Nephron Clin. Pract. 2012, 122, 139–145.
- Chiang, H.H.; Guo, H.R.; Livneh, H.; Lu, M.C.; Yen, M.L.; Tsai, T.Y. Increased risk of progression to dialysis or death in CKD patients with depressive symptoms: A prospective 3-year follow-up cohort study. J. Psychosom. Res. 2015, 79, 228–232.
- Kittiskulnam, P.; Sheshadri, A.; Johansen, K.L. Consequences of CKD on functioning. Semin. Nephrol. 2016, 36, 305–318.
- Fischer, M.J.; Xie, D.; Jordan, N.; Kop, W.J.; Krousel-Wood, M.; Tamura, M.K.; Kusek, J.W.; Ford, V.; Rosen, L.K.; Strauss, L.; et al. CRIC Study Group Investigators CRIC Study Group Investigators. Factors associated with depressive symptoms and use of antidepressant medications among participants in the Chronic Renal Insufficiency Cohort (CRIC) and Hispanic-CRIC Studies. Am. J. Kidney Dis. 2012, 60, 27–38.
- Middleton, R.A.; Allman-Farinelli, M.A. Taste sensitivity is altered in patients with chronic renal failure receiving continuous ambulatory peritoneal dialysis. J. Nutr. 1999, 129, 122–125.
- Kusaba, T.; Mori, Y.; Masami, O.; Hiroko, N.; Adachi, T.; Sugishita, C.; Sonomura, K.; Kimura, T.; Kishimoto, N.; Nakagawa, H.; et al. Sodium restriction improves the gustatory threshold for salty taste in patients with chronic kidney disease. Kidney Int. 2009, 76, 638–643.
- Manley, K.J.; Haryono, R.Y.; Keast, R.S. Taste changes and saliva composition in chronic kidney disease. Ren. Soc. Australas J. 2012, 8, 56–60.
- van der Eijk, I.; Allman Farinelli, M.A. Taste testing in renal patients. J. Ren. Nutr. 1997, 7, 3–9.
- McMahon, E.J.; Campbell, K.L.; Bauer, J.D. Taste perception in kidney disease and relationship to dietary sodium intake. Appetite 2014, 83, 236–241.
