Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Titanium and its alloys are widely employed in the aerospace industry, and their use will increase in the future. Titanium is mainly produced by the Kroll method, but this is expensive and energy-intensive. Therefore, the research of efficient and sustainable methods for its production has become relevant.
- additive manufacturing
- Kroll process
- Titanium
- Titanium alloys
1. Introduction
Titanium and its alloys are known for their lightweight, high specific strength, chemical and corrosion resistance, and favorable biocompatibility, making them suitable for a wide range of applications ranging from aerospace, biomedical, military, petrochemical, and automotive fields [1][2][3]. Despite being dispersed across the Earth’s crust and being challenging to extract, titanium has the potential to emerge as the fourth generation of metal materials after copper, iron, and aluminum [4]. In recent years, the world’s average production of titanium sponge, which is the porous intermediate product derived from the titanium ore (ore is a natural rock or sediment that contains one or more valuable minerals) used to produce titanium ingot (a piece of relatively pure material, usually metal, that is cast into a shape suitable for further processing), has been around 260.000 ton/year [5]. This is significantly less than the 1.88 billion ton/year [6] of crude steel and the 69 million metric ton/year of aluminum [7]. The global market growth of titanium sponge has been steady in recent years and is anticipated to maintain this positive progression until 2030. However, the high cost of this metal limits its applicability mainly in technically demanding sectors where its properties are essential, such as aerospace and aeronautic, military, and some industrial sectors (i.e., oil and gas, chemical processes, power generation, etc.). The high cost of titanium sponge is due to (i) the numerous extraction and production steps; (ii) the high reactivity/affinity with elements such as oxygen and nitrogen; and (iii) the poor machinability caused by the low thermal conductivity. The price of titanium sponge varies greatly among different countries, depending on local demand, supply, and production costs. In recent years, the price of titanium has exceeded 10 €/kg [8]. Table 1 reports a cost comparison of titanium with other materials. The world’s titanium sponge production is concentrated in a few nations, among which the main is China.
| Material | €/Kg |
|---|---|
| Titanium sponge | 10.27 |
| Aluminum ingot | 1.97 |
| Stainless steel | 2.18 |
| Steel | 0.25 |
| Iron | 0.105 |
| Copper | 7.85 |
The titanium sponge is used for the production of titanium ingot, which is manufactured on a large scale across the world by several different companies. Some of the leading companies that are entitled to be key players in the global metal market for titanium are: (i) VSMPO-AVISMA Corporation, which is the world’s largest producer of titanium metal. The company processes the raw materials to produce high-grade titanium metal all over the world. It was incorporated in the year 1993, with its headquarters located in Russia; (ii) TIMET, Sigma Aerospace Metals, Admat Inc., KRONOS, Tronox Incorporated, Castle Metals, ATI Specialty Metals, and Precision Castparts Corporation are key suppliers and manufacturers of titanium metal on a global scale, with headquarters located in the USA; (iii) Toho Titanium is a Japanese metal manufacturing firm that is mainly dedicated to manufacturing titanium on a large scale.
Titanium alloys with strong corrosion resistance are typically utilized in the manufacturing of heat exchangers, tanks, chemical processing, desalination, and power generation plants [11]. In the automotive sector, titanium and alloys are used to produce components for reducing weight and consumption, such as intake and exhaust valves and connecting rods [12]. Takahashi et al. [12] showed that a suitable surface treatment is necessary to minimize wear problems. As far as exhaust valves (exposed to high operative temperatures) are concerned, new alloys with higher heat resistance (close to 800 °C) need to be developed. The biomedical field is another expanding application of titanium alloys. Bombač et al. showed that pure titanium is effective for the production of dental implants and maxillofacial applications [13]. Elias et al. [1] used titanium alloys in the production of cardiac valve prostheses, pacemakers, and artificial hearts. Furthermore, Ti-6Al-4V alloy was used for hip and knee prostheses and trauma fixation devices such as nails, plates, and screws. However, Ti-6Al-4V has some disadvantages: (1) The V and Al elements are toxic to the human body; (2) the alloy has a higher elastic modulus (110 GPa) than that of the bone (18 GPa) [14]. For these reasons, it is necessary to: (1) substitute potential toxic elements (Al and V) with biocompatible alloying elements like Nb, Ta, and Zr; and (2) modulate the elastic modulus through appropriate treatments.
In the aerospace industry, titanium materials are widely used in engine and airframe systems. Its large employment is due to higher operating temperatures (instead of nickel alloys), weight reduction, and corrosion resistance (instead of steel) [15]. Pure titanium can be used for parts subjected to aggressive corrosion, while Ti-6Al-4V is used for parts subjected to high mechanical stresses.
The total amount of titanium alloy utilized for aircraft structural materials is constantly rising. This, in turn, will increase the amount of titanium waste, so solutions to effectively boost the recyclability of scraps will be vital. From a general point of view, the reuse of secondary resources has a certain goal: to maximize the utilization of resources and, meanwhile, reduce potentially dangerous impacts on the environment and people. Recently, the introduction of additive manufacturing (AM) for titanium scraps has demonstrated the potential of these techniques in achieving 100% material usage and 0% waste production. This can significantly contribute to the reduction of costs and energy consumption.
2. Method and Process for Obtaining Titanium and Its Alloys
2.1. Titanium Resources
Titanium is the ninth most abundant element on earth’s crust, with an estimated average amount of 0.6 wt.% [16]. Oxides are the prevalent minerals of titanium due to its great affinity for oxygen: ilmenite (FeTiO3), leucoxene (highly altered ilmenite with a high amount of TiO2), rutile (TiO2), anatase (TiO2), and perovskite (CaTiO3). Currently, the principal source for the titanium industry is the ore, which contains rutile (TiO2) and ilmenite (FeTiO3) [17][18]. The titanium reserves of China rank first in the world, followed by Australia, India, Brazil, and other 9 countries (Figure 1). They account for about 97% of the world’s total reserves [19]. At present, ilmenite deposits are mainly concentrated in China, Australia, India, South Africa, and Brazil, while rutile deposits are distributed in Australia, India, South Africa, and Sierra Leone. Considering the data from the United States Geological Survey, around 2 billion metric tons of titanium ore are available worldwide. They include 700 million metric tons of ilmenite resources and 49 million metric tons of rutile resources. Considering current mining rates of about 7 megatons per year, titanium deposits can last for more than six centuries [20].
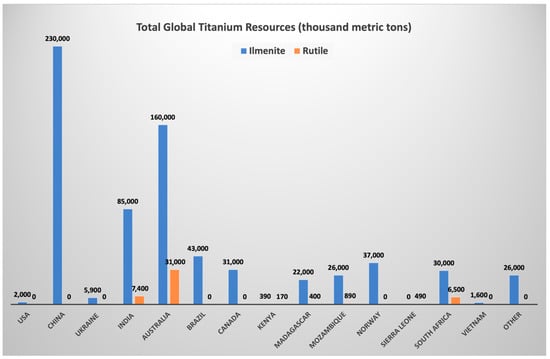
Figure 1. Total Global Titanium Resources [19].
In the ilmenite deposits, the mineral is found in layers as well as in disseminated patterns within igneous anorthosite rock complexes. It is often found coupled with hematite (Fe2O3), forming interposed lamella microstructures, which are characteristic of hemo-ilmenite minerals. Such a kind of mineral is present in Quebec (Canada) [21] or in Tellnes (Norway) [22][23]. The block flow diagram representing ilmenite extraction from Tellnes mines is displayed in Figure 2.
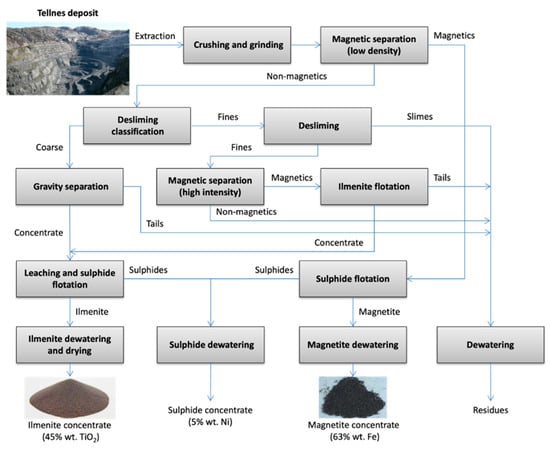
Figure 2. Block flow diagram of the extraction of Ilmenite from Tellnes mines (based on Kingman et al. [24]).
Depending on its geological history, ilmenite includes 40–65 wt.% of TiO2 [25]. Conversely, natural rutile derives mainly from sand deposits and typically contains 92–96 wt.% of TiO2. The main drawback of the rutile is its limited quantity and, consequently, its high cost. Sierra Leone is the region with the highest amount of sand deposits for rutile extraction [26][27].
2.2. Method and Process for Obtaining Titanium Dioxide
Commercial titanium metal is obtained by thermo-chemical reduction techniques, starting with TiCl4. (see next paragraph.) In turn, TiCl4 is produced by the chlorination of TiO2. The major sources for the obtainment of TiO2 are (i) natural rutile and (ii) ilmenite, from which synthetic rutile and titanium slag (i.e., the primary product of ilmenite smelting) can be obtained. When natural rutile is used, a series of concentrations of sands through gravity separation, electrostatic separation to remove non-conducting materials (i.e., zircon materials), and magnetic separation to separate ilmenite are conducted. The block flow diagram for the extraction of rutile from the sand deposit (from which ilmenite is also obtained) is shown in Figure 3.
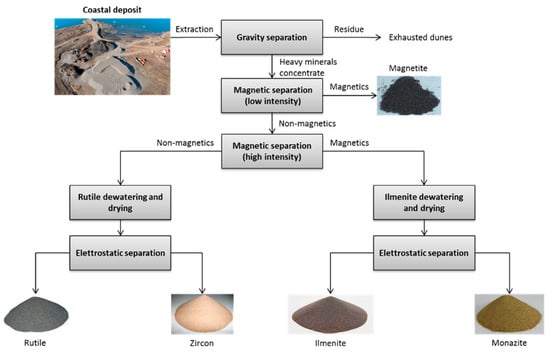
Figure 3. Block flow diagram of the extraction of Ilmenite from the sand deposit (based on Battle et al. [28]).
The rutile concentrate, containing about 95 wt.% of TiO2, is directly used as a raw material [29]. Instead, synthetic rutile is obtained through a combination of thermal oxidation and reduction by roasting sand ilmenite, followed by leaching and physical separation steps for removing the iron. The final product typically contains about 92 wt.% of TiO2. The two most common processes for obtaining this product are the Becher and Benelite processes. However, Murso and Austpac processes are also often used [30].
The Becher process was created and optimized in the late 1960s for the treatment of sand ilmenite in Australia [31], while the Benelite process [32][33] was developed in the United States during the 1970s. In the first one, ilmenite is reduced with coal, and metallic iron is rusted away [34]. In the second, ilmenite is reduced with carbon to convert ferric iron into a ferrous state, and hydrochloric acid is then used to leach the iron [35]. Presently, only two companies are using the Becher process, both operational in Australia, with a production of about 200.000 ton/year of synthetic rutile [36][37]. The Benelite process is rather costly and nowadays is used only in India, with a production of about 150.000 ton/year of synthetic rutile. Finally, high-quality titanium slag is obtained from ilmenite by melting it in an electric arc furnace. The titanium slag, which typically contains about 85–90 wt.% TiO2, has a low percentage of impurities like MgO, CaO, and SiO2. After this step, titanium slag can be processed using a variety of techniques to produce titanium dioxide, such as sulfuric acid leaching, hydrochloric acid leaching, fluoride leaching, ammonia decomposition, and magnetic separation. As reported by Liu et al. [38], almost 200 electric furnaces had been built in China by 2016, with a production of about two million ton/year of titanium slag. Around 60 kton/year of titania slag is also produced in Ukraine, Kazakhstan, Vietnam, and India [39].
The Murso method consists of the oxidation and reduction of ilmenite, followed by leaching with hydrochloric acid. Instead, the Austpac process involves roasting ilmenite ore to magnetize it, separating gangue minerals through magnetic separation, and then leaching with hydrochloric acid [30].
2.3. Titanium Production Process
The affinity of titanium for oxygen and the chemical stability of its mineral form are second only to those of some elements such as Al, Mg, Ca, and some rare-earth metals [40][41]. This makes it difficult and costly to extract and process it into commonly used alloys. In this section, the most commonly used and relevant processes employed for converting TiO2 into titanium sponge are described. The commercial process used today for this conversion is the Kroll process®. The first titanium sponge was manufactured in Japan by this process in 1952 (OSAKA Titanium Technologies Co., Amagasaki, Japan). The Kroll process can be divided into three main sub-processes [42][43]: (1) chlorination and purification where titanium minerals (that, as explained before, are generally natural and synthetic rutile) are chlorinated and distilled to produce pure titanium tetrachloride (TiCl4) (reaction occurs at 1000 °C [44]); (2) reduction and vacuum distillation where titanium tetrachloride is reduced using molten magnesium to produce titanium sponge (reaction takes place in an argon atmosphere at 850–950 °C [44]). Residual magnesium and MgCl2 are removed from the sponge by vacuum distillation; (3) electrolysis, where the magnesium chloride is decomposed into magnesium and chlorine gas. They are reused in the previous processes (reduction and chlorination).
The Kroll process has largely replaced a previous method of producing titanium, known as the Hunter process [45], which used liquid sodium as a reducer. The production of pure ductile metallic titanium was first achieved in industry by the Hunter process. It was invented in 1910 and is like the Kroll process, except for the substitution of sodium as a reductant. The primary obstacle to the Hunter process’s use is the challenge of removing the generated NaCl from the titanium. The vapor pressure of NaCl is lower than that of MgCl2, produced by the Kroll process. As a result, the NaCl is eliminated by aqueous solution leaching. The procedure of extracting the by-product (NaCl) from this aqueous solution needs more energy.
The Hunter process was also modified to incorporate a two-stage reduction. In the first step, TiCl4 and molten sodium react to form TiCl2. After that, this mixture is added to a retort that has enough sodium in it to finish the reaction at a higher temperature. Since titanium subchlorides are soluble in NaCl, it is not feasible to separate the titanium by draining off the NaCl that is formed during the reduction. The final product in the retort contains a mixture of one part titanium and four parts of NaCl. Therefore, compared to a Kroll reaction of comparable size, the amount of titanium generated by the Hunter reaction is substantially less. An example of an effort to create a continuous process based on the Hunter process is the Armstrong process, which attracted a lot of interest and investment during the previous two decades. The Armstrong process uses sodium or magnesium vapor to reduce titanium tetrachloride and may offer advantages in terms of energy efficiency and lower operating temperatures. It has not yet been adopted due to various reasons, such as the need for specialized equipment and potential challenges in scaling up the process to meet industrial demands [25]. In the second half of the last century, many efforts have been conducted to improve the metallothermic reduction process of TiCl4. Table 2 summarizes these attempts.
Table 2. Investigation of the titanium reduction process by metallothermic reduction.
| Reference | Feedstock | Process News | Product |
|---|---|---|---|
| [46] | TiCl4 | Liquid Pb cathode without ceramic diaphragm | Dentrite |
| [47] | Na2TiF6 | Utilizing Ti alloy melt | Liquid alloy |
| [48] | TiO2 | Electrolysis with plasma | |
| [49] | TiCl4 | Reaction with the use of a ceramic diaphragm | Dentrite |
| [50] | TiO2 | Al reduction and EB melting | Deposit |
| [51] | TiCl4 | TiCl4 was injected into liquid Mg | Liquid |
| [52] | TiO2 | Calciothermic reduction with hot-spot cathode | |
| [53] | TiCl4 | Reaction of TiCl4 + Ti → 2 TiCl2 | Sponge |
| [54] | TiCl4 | Reaction with the use of a ceramic diaphragm | Sponge |
| [55] | TiCl3 | Ti-coating and plate deposition | Plate |
| [56] | TiCl4 | TiCl4 was reacted with aerosol Mg | Powder |
| [57] | TiCl4 | Pulse current and rotation electrode | Plate |
These experiments aimed to establish high-speed and continuous responses, which are not present in the Kroll process. Unfortunately, titanium’s high melting point and strong reactivity make such advancements technically challenging. Over the past two decades, alternative processes have been developed, including improved reactor design, omission of cooling steps in TiCl4 reduction with vacuum distillation, and diaphragm-less electrolysis for MgCl2 regeneration (Table 3).
Table 3. Recent developments for new titanium reduction processes [58].
| Institution | Process News | Product |
|---|---|---|
| Aachen University [59] | Aluminothermic reduction of TiO2 | Liquid alloy |
| Armstrong (ITP company) [60] | Sodiothermic reduction of TiCl4 vapor | Powder |
| BHP Billiton [61] | Reduction of TiO2 by Ca in molten CaCl2 | Powder |
| CSIR in South Africa [62] | Electrochemical reduction of Ti slag (in CaF2) | Liquid |
| DMR USA | Aluminothermic reduction of TiO2 | Liquid alloy |
| EMR/MSE Tokyo University [63] | Reduction of TiO2 by liquid Ca alloy | Powder |
| ESR Toyohashi University [64] | Electrolytic reduction of TiO2 | Liquid |
| FFC Cambridge University [65] | Electrochemical reduction of a sintered TiO2 electrode (in CaCl2) | Powder |
| Gtt s.r.l. [66] | Electrochemical reduction of TiCl4 in molten salt | Liquid |
| Idaho research | Reduction of liquid TiCl4 by Mg or Ca | Powder |
| Idaho Ti technology | Reduction of TiCl4 plasma by H2 | Powder |
| JTS (Japan Titanium Society) | Reduction of TiCl4 by liquid Ca | Powder |
| MER Company | Reduction of TiO2 at the anode utilizing the deposition of cathode | Powder |
| MIT | Electrochemical reduction of TiO2 dissolved in molten salts | Liquid |
| OS Kyoto University | Reduction of TiO2 by Ca | Powder |
| PRP Tokyo University | Reduction of TiO2 by Ca vapor | Powder |
| QIT Rio Tinto | Electrochemical reduction of Ti slag | Liquid |
| SRI International | Reduction of TiCl4 by H2 in a fluidized bed | Powder |
| TIRO (CSIRO, Australia) | Reduction of TiCl4 vapor by Mg vapor | Powder |
| University Sci. Tech. of Beijing | Reduction of TiO2 by C | Powder |
| Vartech | Reduction of TiCl4 vapor by H2 | Powder |
A method that has come to the fore in recent years is the hydrogen-assisted Mg reduction (HAMR) process [41][67][68]. In this process, Mg reduction of TiO2 is performed in a hydrogen atmosphere to form titanium hydride (TiH2). Mg is chosen as the reducing agent for its cheapness compared to calcium or sodium. The reduction and deoxygenation steps are used to remove oxygen [69], while a heat treatment is carried out between the two processes to regulate the powder’s specific surface area and particle size [70]. The aim of the last step is the removal of the exceeding hydrogen [71]. The HAMR process allows for an oxygen content lower than 0.15 wt.% in the final product. Figure 4 synthesizes the main steps of the HAMR process.
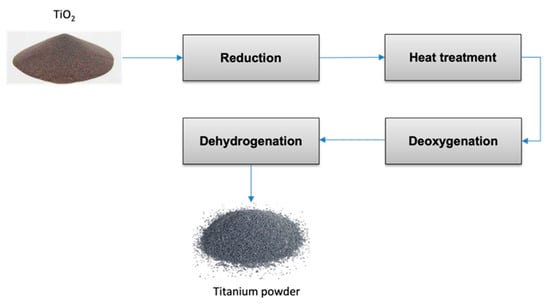
Figure 4. Block flow diagram of the HAMR process.
2.4. Main Production Method of Titanium Alloys
Based on their metallurgical properties, titanium alloys may be categorized into four main groups: α, near α, α–β, and β alloys [72][73]. The alloying elements are classified as neutral, α-stabilizers, or β-stabilizers, and they are used to stabilize the α and the β phases, respectively [74][75]. The α-alloys can be obtained from a α-phase single-solid solution. These alloys show good properties at high temperatures and are mainly used where deformability and corrosion behavior are required [11]. Near-α-alloys are composed of α-phase with less than 10 wt.% of β-phase due to the addition of 1–2 wt.% of β-stabilizers. These alloys, used to produce aeronautical engine components, have good strength and workability due to the presence of the β phase. However, they can be used at a maximum temperature of 500–550 °C [11]. β alloys consist of small quantities of α-stabilizers and 10–15 wt.% of β-stabilizers. The group of α-β alloys includes those that contain 4–16 wt.% of β-stabilizers. As far as the aeronautical field is concerned, the most important alloys are those in the α–β group. Among these, Ti-6Al-4V alloy is the most used, accounting for over 45% of the total titanium production [76]. Elements that raise the transformation temperature (α-stabilizer) are aluminum (Al), oxygen (O), nitrogen (N), and carbon (C). Al is a good α-strengthening element among them, active at both room temperature and elevated temperatures (up to 550 °C). Furthermore, the low density of Al is an important additional advantage. Elements that produce a decrease in the transformation temperature (β-stabilizers) can be divided into β-isomorphous and β-eutectoid elements. The first are molybdenum (Mo), vanadium (V), and niobium (Nb), and promote the stability of β phase along all the composition of the alloy; while the second are chrome (Cr), manganese (Mn), and hydrogen (H), and cause eutectoid transformations of β phase (Figure 5). These elements are soluble with β-titanium and progressively bring the β-to-α transformation up to ambient temperature [75]. While neutral elements, like tin (Sn) and zirconium (Zr), do not have a great influence on transus temperature, α-β titanium alloys are usually processed by conventional (α–β) forging. The first step of the process involves the pure titanium sponge being pre-densified in a hydraulic press to obtain a compact material [77]. Then, this titanium compact is assembled with an electrode for the melting step. α–β stabilizing elements (Al and V, the most common) are added to obtain specific alloy compositions. The pure titanium compact needs to be welded at low pressure with argon in a plasma-welding chamber because of its strong affinity for oxygen. During the process, an arc is ignited between the electrode and the self-consuming electrode, leading to the formation of an ingot. The melting temperature is computer-controlled, and the entire process is carried out in a vacuum. Materials are heated and processed at 40–50 °C below the β transus temperature [78]. The forged alloys have equiaxed microstructures that enhance ductility and thermal stability but reduce high-temperature properties and fracture toughness [78]. α–β alloys are also processed by β-forging processes. In this case, the materials are usually heated above the β transus, and the resulting forged materials have a lamellar microstructure (lamellar α in a transformed β matrix) with higher temperature creep properties, impact toughness, and fracture toughness. Lower thermal stability and ductility are a consequence of this development, though.
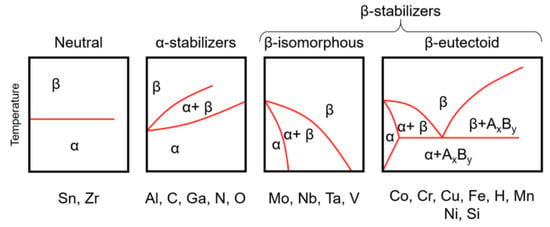
Figure 5. Phase diagram of titanium alloys [79].
In recent decades, new forging strategies have been explored to enhance the mechanical properties of titanium alloys in addition to conventional methods. For instance, advances in the development of dynamic plastic deformation methods to increase the strength of titanium alloys were made by Lu et al. [80] and Fang et al. [81]. Won et al. [82] controlled microstructure by deformation at cryogenic temperatures. Since the treated material’s microstructure was ultra-finely grained, it gained significant strength without compromising its ductility. The production of multiscale structures in a hexagonal closed-packed titanium alloy using bulk nano-structuring has been reported by Zhao et al. [83], with notable improvements in tensile strength and ductility. Regretfully, these processing methods are difficult to successfully apply in real-world applications and are only useful in particular situations.
2.5. Energy Consumption for Titanium Preparation
The literature provides few details regarding the energy consumption of the titanium preparation steps from the perspective of efficiency. Significantly different values can be found for the same step in the processing of the titanium mineral until the Kroll process. Table 4 summarizes the different values for energy consumption as a function of the grade and source of the titanium mineral based on the work of Middlemas and Bradvard [84][85].
Table 4. Energy (MJ/kg) required for TiCl4 preparation [44].
| Raw Mineral | Rutile (98% TiO2) |
Ilmenite Slag (90% TiO2) |
Ilmenite Slag (90% TiO2) |
Ilmenite Slag (90% TiO2) |
Ilmenite Slag (90% TiO2) |
Ilmenite Slag (90% TiO2) |
|---|---|---|---|---|---|---|
| Source | Beach sand (24% TiO2) | Beach sand (1.4% TiO2) | Rocks (20% TiO2) |
Rocks (35% TiO2) |
High Alumina Clay (5% TiO2) |
Soil (0.2% TiO2) |
| Drilling, dredging, and blasting | 0.20 | 1.50 | 0.07 | 0.05 | 0.56 | 13.25 |
| Crushing | 0.20 | 0.40 | 0.45 | 0.30 | 0.80 | 19.10 |
| Gravity concentration | 0.06 | 0.70 | 0.10 | 0 | 0.70 | 6.20 |
| Magnetic concentration | 0.06 | 0.50 | 0.05 | 0 | 0.20 | 4.70 |
| Magnetic separation | 0.14 | 2 | 0.12 | 0 | 0.70 | 16.20 |
| Roasting | 0 | 0 | 0 | 0 | 7.50 | 9 |
| Miscellaneous | 0.14 | 1.15 | 0.10 | 0 | 0.97 | 15.50 |
| Melting of slag | 0 | 18.70 | 19.45 | 24.85 | 23.40 | 23.40 |
| Chlorination | 2.10 | 3.60 | 3.60 | 3.60 | 3.60 | 3.60 |
| TiCl4 purification | 7.20 | 7.90 | 7.90 | 7.90 | 7.90 | 7.95 |
| Total | 10.20 | 36.50 | 31.85 | 36.70 | 46.40 | 118.80 |
Additionally, there is disagreement on the amount of energy needed for each stage of the Kroll process. Peter et al. reported a value of energy consumption for the Kroll process of about 77.4 MJ/kg [86], whereas Kohli et al. fixed this value in the range between 55.44 and 174.24 MJ/kg [87]. By analyzing the individual steps of the Kroll process, it was found that the results are strictly affected by the process condition, the temperature, the efficiency of the used plants, the vacuum level of the distillation, and the required final size of the sponge. For example, the chlorination and purification of TiCl4 require about 3.6 and 7.9 MJ/kg, respectively. The Mg reduction process requires about 36 MJ/kg [87], the vacuum distillation process accounts for values ranging from 64.8 MJ/kg [88] to 133.2 MJ/kg [89], and the electrolysis during magnesium regeneration ranges from 36 to 47.52 MJ/kg [90][91]. Moreover, considering that during the production only 10–50 wt.% of Mg is required to complete the reduction of TiCl4 [92][93], the energy consumption due to the Mg loss is estimated at around 30 MJ/kg [94]. Because of the contamination due to the stainless-steel vessel, a small part of the sponge cannot be employed. It is estimated that there is a residue of 10–20% of titanium sponge in each production [95], causing an energy consumption of 42.96 MJ/kg.
Based on the literature, it is possible to derive an average value of energy consumption f of the whole process of about 258 MJ/kg. This is due to the energy for: the feedstock TiCl4 (129 MJ/kg); the regeneration and loss of the reducing agent (73.2 MJ/kg); Mg reduction (0.9 MJ/kg); vacuum distillation (11.7 MJ/kg); and sponge loss (42.96 MJ/kg). Figure 6 shows the cradle-to-gate lifecycle energy consumption for titanium production.
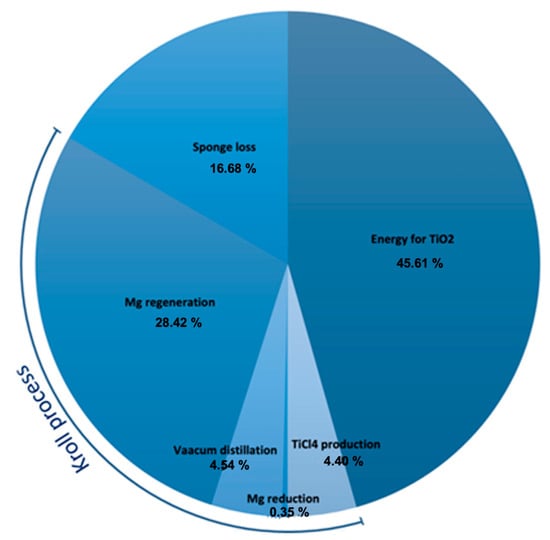
Figure 6. Pie chart for the energy consumption break-down of the titanium production.
The literature does not report extensive data on the energy consumption for other processes of titanium production. In the work of Xia and co-workers [44], the energy consumption of Kroll and HAMR processes was compared step by step. The results indicate that the HAMR process could have a lower consumption of about 25% than that of the Kroll process. Furthermore, in the HAMR process, Mg can be commercially purchased instead of being regenerated. When Mg regeneration is separately considered, the value of energy savings for the HAMR process reaches up to 65%. Regarding the production of titanium alloys, again, few data can be found in the literature. The conventional production of a plate in Ti-6Al-4V alloy requires energy consumption between 582 and 643 MJ/kg [96].
3. Additive Manufacturing of Titanium and Its Alloys
The ASTM F42 Technical Committee defines AM as the “process of joining materials to make objects from three-dimensional (3D) model data, usually layer upon layer, as opposed to subtractive manufacturing methodologies” [97]. This concept includes all those technologies that can build 3D geometries from raw materials [96]. AM has been hailed as a significant industrial technique in recent decades since it may reduce material waste and shorten the manufacturing cycle time. The technology is utilized in various industries like medical, aerospace, military, automotive, oil and gas, and tooling. This is due to its performance gains and ability to fabricate complex net shapes directly from 3D models [98][99][100][101][102].
Production of titanium aircraft obtained by traditional manufacturing has a “buy-to-fly” ratio (the ratio between the mass of the raw material employed to produce a component and the component mass itself) of (12–25):1 [103] with respect to (3–12):1 generally used for AM titanium components [104][105]. This allows faster product development and more efficient use of the raw material. Several additive manufacturing techniques are available, which are classified into different categories based on ASTM [106] standards. Direct energy deposition (DED) and powder bed fusion (PBF) are two of the main methods of AM and are characterized by the way the material is delivered into the fusion bath (Table 5).
Table 5. AM technologies suitable for titanium and its alloy processing [107].
| AM Process | Technology | Description |
|---|---|---|
| Directed Energy Deposition (DED) |
Direct Metal Deposition (DMD) | Laser and metal powder for melting and depositing using a patented close-loop process |
| Laser-Engineered Net Shaping (LENS) | Laser and metal powder for melting and depositing | |
| Direct Manufacturing (DM) | Electron beam and metal wire for melting and depositing | |
| Shaped Metal Deposition or Wire and Arc Additive Manufacturing (WAAM) | Electric arc and metal wire for melting and depositing | |
| Powder Bed Fusion (PBF) | Selective Laser Sintering (SLS) | Laser and metal powder for sintering and bonding |
| Direct Metal Laser Sintering (DMLS) | Laser and metal powder for sintering, melting, and bonding | |
| Laser Melting (LM) | Laser and metal powder for melting and bonding | |
| Selective Laser Melting (SLM) | Laser and metal powder for melting and bonding | |
| Laser CUSING | Laser and metal powder for melting and bonding | |
| Electron Beam Melting (EBM) | Electron beam and metal powder for melting and bonding |
An energy source, usually an electron beam [108], plasma arc [109], or laser [110], is necessary for direct energy deposition together with wire or powder feedstock material. The final properties obtained by the DED method are dependent on the environment (ambient, inert gas, or vacuum), beam-material interactions, feedstock characteristics, and deposition parameters (laser powder, laser scan speed, hatch spacing, powder feed rate, and laser scan strategy) [111]. Among others, the wire and arc additive manufacturing (WAAM) technique is one of the most efficient and successful additive manufacturing processes. WAAM is a direct energy deposition process that utilizes wire feedstock and an electric arc as an energy source [112][113][114]. Many types of arcs, such as plasma arc welding (PAW) [115], gas tungsten arc welding (GTAW) [116], and gas metal arc welding (GMAW) [117], can be utilized as energy sources. WAAM parts often need to be machined to achieve the desired final dimensional tolerances and surface finishes. Studies have demonstrated that WAAM is still more cost-effective than subtractive machining when only an extra machining step is added. This is also linked to the fact that it produces less material waste [118]. Ti-6Al-4V components with mechanical characteristics similar to those obtained by traditional manufacturing procedures can be produced via the WAAM process [114]. However, the control of thermal behavior, which influences the material microstructure, oxidation, and defect generation, is still a challenging task [119]. While DED techniques use a single coaxial nozzle to dispense powder on a substrate, PBF involves spreading powder thinly across the substrate by selectively fusing it with the layer below [120].
The most used PBF processes right now are selective laser melting (SLM) and electron beam melting (EBM) [121]. In SLM, a high-energy laser beam is used to fuse and consolidate small layers of loose powder [122], while in EBM, a high-energy electron beam is used to heat, sinter, and melt the powder.
Compared to EBM, SLM materials tend to be harder and less ductile. Furthermore, unlike SLM machines, the EBM works in a vacuum, thus reducing the risk of oxidation. Another important difference is that the SLM technique can use only metal powder, whereas the EBM technique can use both powder and wire as feedstock material.
Metal wires are easier to find, less expensive, and safer to handle than metal powder. In addition, compared to powder-based systems and other types of metallic AM, wire-feed additive methods provide a greater deposition rate [112]. Nevertheless, it is important to not exceed the increase in the deposition rate because it can cause a reduction in resolution and surface finish.
Despite the disadvantages of the metal powder technique when compared with the metal wire one, it is worth underscoring that the utilization of powder as the starting material allows recycling a significant portion of the material itself, preventing the consumption of precious materials.
Indeed, hundreds of recycling steps are frequently carried out, and sieving operations are often conducted. However, the unmelted metal powder’s form, size distribution, surface morphology, and chemistry may all be progressively altered by repeatedly heating and cooling it in a vacuum. Such deterioration can make powder recycling difficult because the manufactured parts do not meet quality standards. Different recycling approaches were described with the aim of reusing the powders 5 to >30 times [123]. They all concur that the mechanical quality of the printed object starts to decrease significantly after a certain number of recycling steps. Many factors can affect a powder’s capacity to be reused, including:
- (1)
- (2)
-
Contamination of the powder employed. Reusing powder can result in the accumulation of several contaminants derived from manufacturing tools, gaseous elements present during AM, and humidity during storage and/or in powder removal systems.
- (3)
-
Physical characteristics such as density and flowability. The printing process may fail if the powder loses its flowability. The layer packing density of powder will decrease as a result of the growing porosity. This is caused by the keyhole effect or lack of melting, which will also affect the density of products [126]. Consequently, the mechanical properties of the components, such as tensile and fatigue properties, will be affected.
One of the main problems in both traditional and additive manufacturing is the control of the oxygen level, as titanium has a high affinity for it. At low oxygen concentrations, oxygen atoms enter into the octahedral interstitial sites of the α-phase, improving the properties of the solid solution. On the contrary, at high oxygen levels, embrittlement occurs, which reduces the tensile strength, ductility, and fracture toughness of Ti alloys [127]. One method for preventing air contamination is to process the material in an inert gas chamber, under vacuum, or with a localized inert gas shield. Titanium AM processes like EBM and SLM typically operate in vacuum or inert gas chambers, but preventing contamination remains a challenge for out-of-chamber processes like WAAM [128]. In addition to the oxygen contamination, the AM still has some challenges that need to be resolved, such as the high porosity, the high surface roughness, and the propagation of cracks/voids that impact the product quality. All these defects generally increase with increasing recycling numbers [129]. Moreover, the expensive initial investments required for building the industrial implant and the restricted use of large-scale construction are problems to be taken into account [130][131].
Because of these challenges, AM is not yet considered a fully alternative technique to the classical ones. Several studies reported different methods for improving the quality of metal components produced by AM. However, there is still no optimal direction to produce metal AM components. Recently, with the birth and development of artificial intelligence, new opportunities have been created to face the AM problems [132][133][134].
This entry is adapted from the peer-reviewed paper 10.3390/su16010330
References
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M.A. Biomedical Applications of Titanium and Its Alloys. JOM 2008, 60, 46–49.
- Jackson, M.J.; Kopac, J.; Balazic, M.; Bombac, D.; Brojan, M.; Kosel, F. Titanium and Titanium Alloy Applications in Medicine. In Surgical Tools and Medical Devices; Ahmed, W., Jackson, M.J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 475–517. ISBN 978-3-319-33489-9.
- Blanco, D.; Rubio, E.M.; Marín, M.M.; Davim, J.P. Advanced Materials and Multi-Materials Applied in Aeronautical and Automotive Fields: A Systematic Review Approach. Procedia CIRP 2021, 99, 196–201.
- Feng, Q.; Lv, M.; Mao, L.; Duan, B.; Yang, Y.; Chen, G.; Lu, X.; Li, C. Research Progress of Titanium Sponge Production: A Review. Metals 2023, 13, 408.
- Garside, M. Production Volume of Titanium Sponge Worldwide in 2022, by Country 2023. Available online: https://www.statista.com/statistics/1394487/global-titanium-sponge-production-by-country (accessed on 11 September 2023).
- Statista Research Department World Crude Steel Production. 2022. Available online: https://www.statista.com/statistics/267264/world-crude-steel-production/ (accessed on 16 October 2023).
- International Aluminium Institute Primary Aluminium Production—International Aluminium Institute 2021. Available online: https://international-aluminium.org/statistics/primary-aluminium-production (accessed on 11 September 2023).
- Takeda, O.; Okabe, T.H. Current Status of Titanium Recycling and Related Technologies. JOM 2019, 71, 1981–1990.
- Statista Research Department Copper Price. 2023. Available online: https://www.statista.com/statistics/673494/monthly-prices-for-copper-worldwide/ (accessed on 16 October 2023).
- Statista Research Department Iron Ore Price Monthly. 2023. Available online: https://www.statista.com/statistics/300419/monthly-iron-ore-prices/ (accessed on 16 October 2023).
- Veiga, C.; Davim, J.P.; Loureiro, A. Properties and Applications of Titanium Alloys: A Brief Review. Rev. Adv. Mater. Sci. 2012, 32, 133–148.
- Takahashi, K.; Mori, K.; Takebe, H. Application of Titanium and Its Alloys for Automobile Parts. MATEC Web Conf. 2020, 321, 02003.
- Bombač, D.; Brojan, M.; Fajfar, P.; Kosel, F.; Turk, R. Review of Materials in Medical Applications. Mater. Geoenviron. 2007, 54, 471–499.
- Katti, K.S. Biomaterials in Total Joint Replacement. Colloids Surf. B Biointerfaces 2004, 39, 133–142.
- Boyer, R.R. An Overview on the Use of Titanium in the Aerospace Industry. Mater. Sci. Eng. A 1996, 213, 103–114.
- Jin, T.; Costa, M.; Chen, X. Chapter 34—Titanium. In Handbook on the Toxicology of Metals, 5th ed.; Nordberg, G.F., Costa, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 857–868. ISBN 978-0-12-822946-0.
- Zhai, J.; Chen, P.; Sun, W.; Chen, W.; Wan, S. A Review of Mineral Processing of Ilmenite by Flotation. Miner. Eng. 2020, 157, 106558.
- Parrino, F.; Palmisano, L. Titanium Dioxide (TiO2) and Its Applications; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-820434-4.
- U.S. Geological Survey Mineral Commodity Summaries 2022—Titanium and Titanium Dioxide 2022. Available online: https://pubs.usgs.gov/publication/mcs2022 (accessed on 11 September 2023).
- Bedinger, G.M. Titanium Mineral Concentrates 2018. U.S. Geological Survey, 2018, Mineral Commodity Summaries 2018. Available online: https://pubs.usgs.gov/publication/70194932 (accessed on 11 September 2023).
- Charlier, B.; Namur, O.; Malpas, S.; De Marneffe, C.; Duchesne, J.-C.; Auwera, J.V.; Bolle, O. Origin of the Giant Allard Lake Ilmenite Ore Deposit (Canada) by Fractional Crystallization, Multiple Magma Pulses and Mixing. Lithos 2010, 117, 119–134.
- Charlier, B.; Skår, Ø.; Korneliussen, A.; Duchesne, J.-C.; Vander Auwera, J. Ilmenite Composition in the Tellnes Fe–Ti Deposit, SW Norway: Fractional Crystallization, Postcumulus Evolution and Ilmenite–Zircon Relation. Contrib Miner. Pet. 2007, 154, 119–134.
- Fang, Z.Z.; Froes, F.; Zhang, Y. Extractive Metallurgy of Titanium: Conventional and Recent Advances in Extraction and Production of Titanium Metal; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-0-12-817201-8.
- Kingman, S.W.; Corfield, G.M.; Rowson, N.A. Effects of Microwave Radiation Upon the Mineralogy and Magnetic Processing of a Massive Norwegian Ilmenite Ore. Magn. Electr. Sep. 1999, 9, 131–148.
- Zhang, W.; Zhu, Z.; Cheng, C.Y. A Literature Review of Titanium Metallurgical Processes. Hydrometallurgy 2011, 108, 177–188.
- Pohl, W.L. Economic Geology, Principles and Practice: Metals, Minerals, Coal and Hydrocarbons: An Introduction to Formation and Sustainable Exploitation of Mineral Deposits; Wiley-Blackwell: Oxford, UK, 2011.
- Force, E.R. Geology of Titanium-Mineral Deposits; The Geological Society of America: Boulder, CO, USA, 1991.
- Battle, T.P.; Nguyen, D.; Reeves, J.W. The Processing of Titanium-Containing Ores. In Extractive Metallurgy of Copper, Nickel and Cobalt, Fundamental Aspects; Reddy, R.G., Weizenbach, R.N., Eds.; The Minerals, Metals and Materials Society: Warrendale, PA, USA, 1993; Volume I; pp. 925–943.
- Kahn, J.A. Non-Rutile Feedstocks for the Production of Titanium. JOM 1984, 36, 33–38.
- Xiang, J.; Pei, G.; Lv, W.; Liu, S.; Lv, X.; Qiu, G. Preparation of Synthetic Rutile from Reduced Ilmenite through the Aeration Leaching Process. Chem. Eng. Process. Process Intensif. 2020, 147, 107774.
- Fromanek, L.; Lomert, H.; Beyzavi, A.N. Synthetic Rutile Manufacture by the SR/RNBecher Process. In Heavy Minerals 1997; Robinson, R.E., Ed.; Southern African Institute of Mining and Metallurgy: Johannesburg, South Africa, 1997; pp. 161–168.
- Iammartino, N.R. Beneficiated-Ilmenite Process Recycles HCl Leach Liquor. Chem. Eng. 1976, 83, 100–101.
- Mackey, T.S. Upgrading Ilmenite into a High-Grade Synthetic Rutile. JOM 1994, 46, 59–64.
- El-Hazek, N.; Lasheen, T.A.; El-Sheikh, R.; Zaki, S.A. Hydrometallurgical Criteria for TiO2 Leaching from Rosetta Ilmenite by Hydrochloric Acid. Hydrometallurgy 2007, 87, 45–50.
- Kurniawan, M.R.; Imami, T.G.; Ichlas, Z.T.; Hidayat, T.; Mubarok, M.Z. Production of Synthetic Rutile from Tin Ore Beneficiation Byproduct through Preoxidation and Reductive Leaching in Hydrochloric Acid. Sci. Rep. 2022, 12, 9092.
- Strange, N.R. Synthetic Rutile Production at Westralian Sands Ltd. In The Sir Maurice Mawby Memorial Volume, 2nd ed.; Hamilton, J.K., Ed.; The Australasian Institute of Mining and Metallurgy: Carlton, VIC, Australia, 1993; pp. 1308–1311.
- Mackowski, S.J.; Reaveley, B.J. Synthetic Rutile Production at Tiwest Joint Venture. In The Sir Maurice Mawby Memorial Volume, 2nd ed.; Hamilton, Ed.; The Australasian Institute of Mining and Metallurgy: Carlton, VIC, Australia, 1993; pp. 1304–1308.
- Liu, Q.; Baker, P.; Zhao, H. Titanium Sponge Production Technology in China; Woodfield, A., Ed.; he Minerals, Metals & Materials Society: Warrandale, PA, USA, 2016; pp. 177–182.
- McCoy, D. High Grade Titanium Feedstocks under Pressure. In Proceedings of the Titanium USA 2018 Conference, Las Vegas, NV, USA, 7–10 October 2018.
- Reed, T.B.; Klerer, J. Free Energy of Formation of Binaray Compounds: An Atlas of Charts for High-Temperature Chemical Calculations. J. Electrochem. Soc. 1972, 119, 329Ca.
- Fang, Z.Z.; Paramore, J.D.; Sun, P.; Chandran, K.S.R.; Zhang, Y.; Xia, Y.; Cao, F.; Koopman, M.; Free, M. Powder Metallurgy of Titanium—Past, Present, and Future. Int. Mater. Rev. 2018, 63, 407–459.
- van Vuuren, D.S. A Critical Evaluation of Processes to Produce Primary Titanium. J. S. Afr. Inst. Min. Metall. 2009, 109, 455–461.
- Qian, M.; Froes, F.H. Titanium Powder Metallurgy: Science, Technology and Applications; Butterworth-Heinemann: Oxford, UK, 2015.
- Xia, Y.; Lefler, H.D.; Fang, Z.Z.; Zhang, Y.; Sun, P. Chapter 17—Energy Consumption of the Kroll and HAMR Processes for Titanium Production. In Extractive Metallurgy of Titanium; Fang, Z.Z., Froes, F.H., Zhang, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 389–410. ISBN 978-0-12-817200-1.
- Wood, R.A.; Poulsen, E.R.; Froes, F.H. (Sam) History and Extractive Metallurgy. In Titanium: Physical Metallurgy, Processing, and Applications; Froes, F.H., Ed.; ASM International: Detroit, MI, USA, 2015; pp. 1–27.
- Ginatta, M.V. Method of Producing Metals by Cathodic Dissolution of Their Compounds 1983. CA Patent No. 1215935A, 30 December 1986.
- Hard, R.A.; Priet, M.A. Process for Making Titanium Metal from Titanium Ore. U.S. Patent No. 4390365, 28 June 1983.
- Larson, H.R.; Eagar, T.W. The Plasma-Enabled Recovery of Titanium by the Electrolysis of Titanate Slags. JOM 1998, 50, 56–57.
- Leone, O.Q.; Knudsen, H.; Couch, D. High-Purity Titanium Electrowon from Titanium Tetrachloride. JOM 1967, 19, 18–23.
- Maeda, M.; Yahata, T.; Mitugi, K.; Ikeda, T. Aluminothermic Reduction of Titanium Oxide. Mater. Trans. JIM 1993, 34, 599–603.
- Ogasawara, T. Research for New Titanium Extraction Process. Titan. Jpn. 1995, 43, 23–27.
- Takeo, O.; Hiroshi, I. Reduction of Titanium Dioxide by Calcium in Hot Cathode Spot. Mem. Fac. Eng. Nagoya Univ. 1968, 19, 164–166.
- Opie, R.W. Basket-Cathode Electrolytic Cell for Production of Titanium Metal. Trans. AIME 1960, 218, 646–649.
- Rand, M.J.; Reimert, L.J. Electrolytic Titanium from TiCl4: I. Operation of a Reliable Laboratory Cell. J. Electrochem. Soc. 1964, 111, 429.
- Robin, A.; De Lepinay, J.; Barbier, M.J. Electrolytic Coating of Titanium onto Iron and Nickel Electrodes in the Molten LiF + NaF + KF Eutectic. J. Electroanal. Chem. Interfacial Electrochem. 1987, 230, 125–141.
- Takeuchi, S.; Kurosawa, T.; Tezuka, M. Thermodynamical Studies and Preliminary Experiments on Production of Ti by Reaction in Gaseous Phase. J. Jpn. Inst. Met. 1959, 23, 625–629.
- Tokumoto, S. Invention of Electrodeposition Process for Smooth Titanium Film. Titan. Jpn. 1959, 7, 11.
- Okabe, T.H.; Takeda, O. Chapter 5—Fundamentals of Thermochemical Reduction of TiCl4. In Extractive Metallurgy of Titanium; Fang, Z.Z., Froes, F.H., Zhang, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 65–95. ISBN 978-0-12-817200-1.
- Stoephasius, J.C.; Friedrich, B.; Hammerschmidt, J. A New Processing Route for Titanium Alloys by Aluminothermic Reduction of Titanium Dioxide and Refining by ESR. In Proceedings of the 10th World Conference on Titanium, Hamburg, Germany, 13–18 July 2003.
- Araci, K.; Mangabhai, D.; Akhtar, K. 9—Production of Titanium by the Armstrong Process®. In Titanium Powder Metallurgy; Qian, M., Froes, F.H., Eds.; Butterworth-Heinemann: Boston, MA, USA, 2015; pp. 149–162. ISBN 978-0-12-800054-0.
- Suzuki, R.O. Direct Reduction Processes for Titanium Oxide in Molten Salt. JOM 2007, 59, 68–71.
- Kraft, E.H. Summary of Emerging Titanium Cost Reduction Technologies 2004; EHK Technologies-for ORNL: Vancouver, WA, USA, 2004; pp. 1–59.
- Park, I.; Abiko, T.; Okabe, T.H. Production of Titanium Powder Directly from TiO2 in CaCl2 through an Electronically Mediated Reaction (EMR). J. Phys. Chem. Solids 2005, 66, 410–413.
- Takenaka, T.; Matsuo, H.; Sugawara, M.; Kawakami, M. High Temperature Electrolysis of Ti and Its Alloys with a DC-ESR Unit. Key Eng. Mater. 2010, 436, 85–91.
- Hu, D.; Dolganov, A.; Ma, M.; Bhattacharya, B.; Bishop, M.T.; Chen, G.Z. Development of the Fray-Farthing-Chen Cambridge Process: Towards the Sustainable Production of Titanium and Its Alloys. JOM 2018, 70, 129–137.
- Ginatta, M.V. Titanium Electrowinning; International Symposium on Ionic Liquids in Honour of Marcelle Gaune-Escard Carry le: Rouet, France, 2023.
- Zhang, Y.; Fang, Z.Z.; Xia, Y.; Huang, Z.; Lefler, H.; Zhang, T.; Sun, P.; Free, M.L.; Guo, J. A Novel Chemical Pathway for Energy Efficient Production of Ti Metal from Upgraded Titanium Slag. Chem. Eng. J. 2016, 286, 517–527.
- Xia, Y.; Fang, Z.Z.; Zhang, Y.; Lefler, H.; Zhang, T.; Sun, P.; Huang, Z. Hydrogen Assisted Magnesiothermic Reduction (HAMR) of Commercial TiO2 to Produce Titanium Powder with Controlled Morphology and Particle Size. Mater. Trans. 2017, 58, 355–360.
- Zhang, Y.; Fang, Z.Z.; Sun, P.; Zheng, S.; Xia, Y.; Free, M. A Perspective on Thermochemical and Electrochemical Processes for Titanium Metal Production. JOM 2017, 69, 1861–1868.
- Zhang, Y.; Fang, Z.Z.; Xia, Y.; Sun, P.; Van Devener, B.; Free, M.; Lefler, H.; Zheng, S. Hydrogen Assisted Magnesiothermic Reduction of TiO2. Chem. Eng. J. 2017, 308, 299–310.
- Xia, Y.; Lefler, H.D.; Zhang, Y.; Sun, P.; Fang, Z.Z. Chapter 9—Hydrogen Assisted Magnesiothermic Reduction (HAMR) of TiO2 to Produce Titanium Metal Powder. In Extractive Metallurgy of Titanium; Fang, Z.Z., Froes, F.H., Zhang, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 165–179. ISBN 978-0-12-817200-1.
- Blenkinsop, P.A. Titanium Alloys. Advances in Alloys, Processes, Products and Applications. J. Phys. IV Fr. 1993, 3, C7–C169.
- Machado, A.R.; Wallbank, J. Machining of Titanium and Its Alloys—A Review. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 1990, 204, 53–60.
- Duncan, R.M.; Blenkinsop, P.A.; Goosey, R.E. Titanium Alloys. In The Development of Gas Turbine Materials; Meetham, G.W., Ed.; Springer: Dordrecht, The Netherlands, 1981; pp. 63–87. ISBN 978-94-009-8111-9.
- Eylon, D.; Fujishiro, S.; Postans, P.J.; Froes, F.H. High-Temperature Titanium Alloys—A Review. JOM 1984, 36, 55–62.
- Wood, R.A.; Favor, R.J. Titanium Alloys Handbook; Air Force Materials Laboratory: Wright-Patterson Air Force Base, OH, USA, 1972.
- Sibum, H. Titanium and Titanium Alloys—From Raw Material to Semi-Finished Products. Adv. Eng. Mater. 2003, 5, 393–398.
- Froes, F.H.; Caplan, I.L. (Eds.) Titanium ’92, Science and Technology; Metallurgical Society of AIME: San Diego, CA, USA, 1992.
- Gialanella, S.; Malandruccolo, A. Titanium and Titanium Alloys. In Aerospace Alloys; Gialanella, S., Malandruccolo, A., Eds.; Topics in Mining, Metallurgy and Materials Engineering; Springer International Publishing: Cham, Switzerland, 2020; pp. 129–189. ISBN 978-3-030-24440-8.
- Lu, K.; Lu, L.; Suresh, S. Strengthening Materials by Engineering Coherent Internal Boundaries at the Nanoscale. Science 2009, 324, 349–352.
- Fang, T.H.; Li, W.L.; Tao, N.R.; Lu, K. Revealing Extraordinary Intrinsic Tensile Plasticity in Gradient Nano-Grained Copper. Science 2011, 331, 1587–1590.
- Won, J.W.; Lee, J.H.; Jeong, J.S.; Choi, S.-W.; Lee, D.J.; Hong, J.K.; Hyun, Y.-T. High Strength and Ductility of Pure Titanium via Twin-Structure Control Using Cryogenic Deformation. Scr. Mater. 2020, 178, 94–98.
- Zhao, S.; Zhang, R.; Yu, Q.; Ell, J.; Ritchie, R.O.; Minor, A.M. Cryoforged Nanotwinned Titanium with Ultrahigh Strength and Ductility. Science 2021, 373, 1363–1368.
- Middlemas, S.; Fang, Z.Z.; Fan, P. Life Cycle Assessment Comparison of Emerging and Traditional Titanium Dioxide Manufacturing Processes. J. Clean. Prod. 2015, 89, 137–147.
- Bravard, J.C.; Flora, H.B.; Portal, C. Energy Expenditures Associated with the Production and Recycle of Metals; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1972.
- Peter, W.H.; Yamamoto, Y.; Chen, W.; Dehoff, R.R.; Nunn, S.D.; Sabau, A.S.; Kiggans, J.O., Jr.; Muth, T.R.; Daehn, G.; Tallman, C.; et al. Near Net Shape Manufacturing of New, Low Cost Titanium Powders for Industry; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2013; pp. 1–98.
- Kohli, R. Production of Titanium from Ilmenite: A Review; Lawrence Berkeley National Lab (LBNL): Berkeley, CA, USA, 1981.
- Baroch, C.T.; Kaczmarek, T.B.; Barnes, W.D.; Galloway, L.W.; Mark, W.M.; Lee, G.A. Titanium Plant at Boulder City, Nev.: Its Design and Operation; Bureau of Mines: Washington, DC, USA, 1955.
- Ishizuka, H. Osaka Special Steel Plant: Japanese Plant to Produce Ti. Eng. Min. J. 1951, 52, 104.
- Evans, J.W. The Evolution of Technology for Light Metals over the Last 50 Years: Al, Mg, and Li. JOM 2007, 59, 30–38.
- Sun, Z.; Cai, L.; Liu, C.; Lu, G.; Yu, J. Analysis for Effects of Electrolyte Level on Energy Consumption in Magnesium Electrolysis by Finite Element Method. Can. J. Chem. Eng. 2017, 95, 648–655.
- Mishra, B. Review of Extraction, Processing, Properties, and Applications of Reactive Metals: 1999 TMS Annual Meeting, San Diego, CA, 28 February–15 March 1999; John Wiley & Sons: Hoboken, NJ, USA, 2013; ISBN 978-1-118-78800-4.
- Nagesh, C.R.V.S.; Rao, C.S.; Ballal, N.B.; Rao, P.K. Mechanism of Titanium Sponge Formation in the Kroll Reduction Reactor. Metall. Mater. Trans. B 2004, 35, 65–74.
- van der Voet, E.; Salminen, R.; Eckelman, M.; Mudd, G.; Norgate, T.; Hisschier, R. Environmental Risks and Challenges of Anthropogenic Metals Flows and Cycles, A Report of the Working Group on the Global Metal Flows to the International Resource Panel; United Nations Environment Programme (UNEP): Nairobi, Kenya, 2013.
- Taninouchi, Y.; Hamanaka, Y.; Okabe, T.H. Chlorination-Volatilization Behavior of Titanium Metal Scraps during Recycling Using Reaction-Mediating Molten Salt. Mater. Trans. 2016, 57, 1309–1318.
- Baumers, M.; Tuck, C.; Wildman, R.; Ashcroft, I.; Hague, R. Shape Complexity and Process Energy Consumption in Electron Beam Melting: A Case of Something for Nothing in Additive Manufacturing? J. Ind. Ecol. 2017, 21, S157–S167.
- ASTM F2792–10; Standard Terminology for Additive Manufacturing Technologies. ASTM International: West Conshohocken, PA, USA, 2010.
- Herzog, D.; Seyda, V.; Wycisk, E.; Emmelmann, C. Additive Manufacturing of Metals. Acta Mater. 2016, 117, 371–392.
- Guo, N.; Leu, M.C. Additive Manufacturing: Technology, Applications and Research Needs. Front. Mech. Eng. 2013, 8, 215–243.
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping and Direct Digital Manufacturing, 2nd ed.; Springer: New York, NY, USA; Berlin/Heidelberg, Germany; Dodrecht, The Netherlands; London, UK, 2015; ISBN 978-1-4939-2112-6.
- Kannan, G.B.; Rajendran, D.K. A Review on Status of Research in Metal Additive Manufacturing; Springer: Singapore, 2017; pp. 95–100.
- Attar, H.; Kent, D. Titanium Alloys for Biomedical Implants and Devices; MDPI: Basel, Switzerland, 2021; ISBN 978-3-0365-0002-7.
- Huang, R.; Riddle, M.; Graziano, D.; Warren, J.; Das, S.; Nimbalkar, S.; Cresko, J.; Masanet, E. Energy and Emissions Saving Potential of Additive Manufacturing: The Case of Lightweight Aircraft Components. J. Clean. Prod. 2016, 135, 1559–1570.
- Shapiro, A.A.; Borgonia, J.P.; Chen, Q.N.; Dillon, R.P.; McEnerney, B.; Polit-Casillas, R.; Soloway, L. Additive Manufacturing for Aerospace Flight Applications. J. Spacecr. Rocket. 2016, 53, 952–959.
- Allen, J. An Investigation into the Comparative Costs of Additive Manufacture vs. Machine from Solid for Aero Engine Parts; 2006. Available online: https://www.sto.nato.int/publications/STO%20Meeting%20Proceedings/RTO-MP-AVT-139/MP-AVT-139-17.pdf (accessed on 11 September 2023).
- ASTM 52900; Standard Terminology for Additive Manufacturing—General Principles—Terminology. ISO/ASTM International: Geneva, Switzerland, 2015.
- Dutta, B.; Froes, F.H. The Additive Manufacturing (AM) of Titanium Alloys. Met. Powder Rep. 2017, 72, 96–106.
- Adebayo, A. Characterisation of Integrated WAAM and Machining Processes; Cranfield University: Cranfield, UK, 2013.
- Almeida, P.M.S. Process Control and Development in Wire and Arc Additive Manufacturing; Cranfield University: Cranfield, UK, 2012.
- Ding, Y.; Akbari, M.; Kovacevic, R. Process Planning for Laser Wire-Feed Metal Additive Manufacturing System. Int. J. Adv. Manuf. Technol. 2018, 95, 355–365.
- Svetlizky, D.; Das, M.; Zheng, B.; Vyatskikh, A.L.; Bose, S.; Bandyopadhyay, A.; Schoenung, J.M.; Lavernia, E.J.; Eliaz, N. Directed Energy Deposition (DED) Additive Manufacturing: Physical Characteristics, Defects, Challenges and Applications. Mater. Today 2021, 49, 271–295.
- Ding, D.; Pan, Z.; Cuiuri, D.; Li, H. Wire-Feed Additive Manufacturing of Metal Components: Technologies, Developments and Future Interests. Int. J. Adv. Manuf. Technol. 2015, 81, 465–481.
- Busachi, A.; Erkoyuncu, J.; Colegrove, P.; Martina, F.; Ding, J. Designing a WAAM Based Manufacturing System for Defence Applications. Procedia CIRP 2015, 37, 48–53.
- Williams, S.W.; Martina, F.; Addison, A.C.; Ding, J.; Pardal, G.; Colegrove, P. Wire + Arc Additive Manufacturing. Mater. Sci. Technol. 2016, 32, 641–647.
- Stavinoha, J.N. Investigation of Plasma Arc Welding as a Method for the Additive Manufacturing of Ti-6Al-4V Alloy Components; Montana Tech of The University of Montana: Butte, MT, USA, 2012.
- Antonysamy, A.A. Microstructure, Texture and Mechanical Property Evolution During Additive Manufacturing Of Ti6Al4V Alloy for Aerospace Applications; The University of Manchester: Manchester, UK, 2012.
- Haselhuhn, A.S. Design for Low-Cost Gas Metal Arc Weld-Based Aluminum 3-D Printing; Michigan Technological University: Houghton, MI, USA, 2016.
- Zhai, Y. Early Cost Estimation for Additive Manufacture; Cranfield University: Cranfield, UK, 2012.
- Åkerfeldt, P.; Antti, M.-L.; Pederson, R. Influence of Microstructure on Mechanical Properties of Laser Metal Wire-Deposited Ti-6Al-4V. Mater. Sci. Eng. A 2016, 674, 428–437.
- Beese, A.M.; Carroll, B.E. Review of Mechanical Properties of Ti-6Al-4V Made by Laser-Based Additive Manufacturing Using Powder Feedstock. JOM 2016, 68, 724–734.
- Gong, H.; Rafi, K.; Gu, H.; Starr, T.; Stucker, B. Analysis of Defect Generation in Ti–6Al–4V Parts Made Using Powder Bed Fusion Additive Manufacturing Processes. Addit. Manuf. 2014, 1–4, 87–98.
- Gu, D.; Wang, H.; Zhang, G. Selective Laser Melting Additive Manufacturing of Ti-Based Nanocomposites: The Role of Nanopowder. Metall. Mater. Trans. A 2014, 45, 464–476.
- Tang, H.P.; Qian, M.; Liu, N.; Zhang, X.Z.; Yang, G.Y.; Wang, J. Effect of Powder Reuse Times on Additive Manufacturing of Ti-6Al-4V by Selective Electron Beam Melting. JOM 2015, 67, 555–563.
- ASTM F3001–14; Standard Specification for Additive Manufacturing Titanium-6 Aluminum-4 Vanadium ELI (Extra Low Interstitial) with Powder Bed Fusion. ASTM International: West Conshohocken, PA, USA, 2014.
- ASTM F2924-14; Standard Specification for Additive Manufacturing Titanium-6 Aluminum-4 Vanadium with Powder Bed Fusion. ASTM International: West Conshohocken, PA, USA, 2014.
- Cunningham, R.; Nicolas, A.; Madsen, J.; Fodran, E.; Anagnostou, E.; Sangid, M.D.; Rollett, A.D. Analyzing the Effects of Powder and Post-Processing on Porosity and Properties of Electron Beam Melted Ti-6Al-4V. Mater. Res. Lett. 2017, 5, 516–525.
- Iantaffi, C.; Leung, C.L.A.; Chen, Y.; Guan, S.; Atwood, R.C.; Lertthanasarn, J.; Pham, M.-S.; Meisnar, M.; Rohr, T.; Lee, P.D. Oxidation Induced Mechanisms during Directed Energy Deposition Additive Manufactured Titanium Alloy Builds. Addit. Manuf. Lett. 2021, 1, 100022.
- Bermingham, M.J.; Thomson-Larkins, J.; St John, D.H.; Dargusch, M.S. Sensitivity of Ti-6Al-4V Components to Oxidation during out of Chamber Wire + Arc Additive Manufacturing. J. Mater. Process. Technol. 2018, 258, 29–37.
- Chekotu, J.; Groarke, R.; O’Toole, K.; Brabazon, D. Advances in Selective Laser Melting of Nitinol Shape Memory Alloy Part Production. Materials 2019, 12, 809.
- Martin, A.A.; Calta, N.P.; Khairallah, S.A.; Wang, J.; Depond, P.J.; Fong, A.Y.; Thampy, V.; Guss, G.M.; Kiss, A.M.; Stone, K.H.; et al. Dynamics of Pore Formation during Laser Powder Bed Fusion Additive Manufacturing. Nat. Commun. 2019, 10, 1987.
- Pérez, M.; Carou, D.; Rubio, E.M.; Teti, R. Current Advances in Additive Manufacturing. Procedia CIRP 2020, 88, 439–444.
- Xiong, Y.; Tang, Y.; Zhou, Q.; Ma, Y.; Rosen, D.W. Intelligent Additive Manufacturing and Design: State of the Art and Future Perspectives. Addit. Manuf. 2022, 59, 103139.
- Qin, J.; Hu, F.; Liu, Y.; Witherell, P.; Wang, C.C.L.; Rosen, D.W.; Simpson, T.W.; Lu, Y.; Tang, Q. Research and Application of Machine Learning for Additive Manufacturing. Addit. Manuf. 2022, 52, 102691.
- Parvanda, R.; Kala, P. Trends, Opportunities, and Challenges in the Integration of the Additive Manufacturing with Industry 4.0. Prog. Addit. Manuf. 2023, 8, 587–614.
This entry is offline, you can click here to edit this entry!
