Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
High-sensitivity cardiac troponin (hs-cTn) assays have revolutionized the assessment of myocardial stress and injury due to their superior sensitivity and accuracy in detecting even minor cardiac damage. However, hs-cTn is often included in the initial blood draw for baseline assessment in such patients and has prognostic value in predicting in-hospital and long-term mortality.
- acute myocardial infarction
- cardiac biomarkers
- high-sensitivity cardiac troponins
1. Introduction
Cardiovascular diseases are the leading cause of death globally. In Singapore, ischemic heart diseases (IHDs) accounted for 19.7% of total deaths in 2022 [1]. A significant number of patients with acute coronary syndrome (ACS) often fail to receive timely treatment owing to incorrect preliminary diagnosis [2]. Most cases of acute myocardial infarction (AMI) present with chest pain, and a proper, rapid diagnostic assessment is essential so that early and effective treatment can be implemented. Similarly, prompt the rule-out of an AMI would avoid unnecessary hospital admissions.
In the initial evaluation of suspected ACS clinical assessment, 12-lead electrocardiogram (ECG), and cardiac troponins (cTn) play a crucial role [3][4]. ST-elevation MI (STEMI) requires immediate reperfusion therapy while very high-risk non-STEMI needs immediate invasive strategy (angiography ± percutaneous coronary intervention). For non-STEMI with high-risk features, angiography within 24 h can be considered. This algorithm is illustrated in Figure 1. Over the recent years, high-sensitivity cardiac troponin (hs-cTn) assays have revolutionized the assessment of myocardial stress and injury due to their superior sensitivity and accuracy in detecting even minor cardiac damage [5]. Patients with STEMI or very high-risk non-STEMI do not need troponin for diagnosis, but their management should not be delayed by waiting for troponin results to be available. However, hs-cTn is often included in the initial blood draw for baseline assessment in such patients and has prognostic value in predicting in-hospital and long-term mortality. For the remining patients with suspected ACS, hs-cTn is pivotal for confirmation of diagnosis, risk assessment/prognostication and subsequent management.
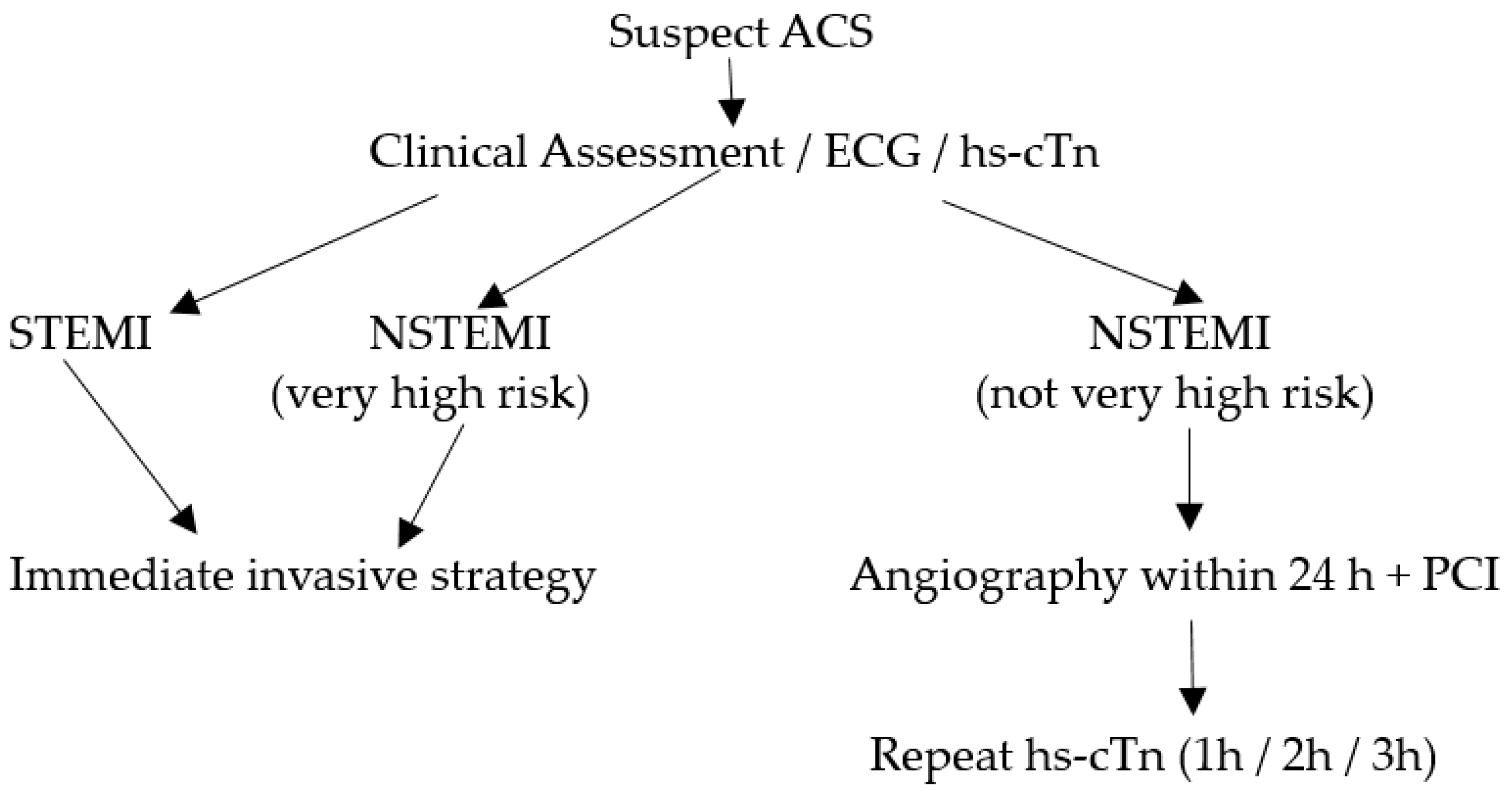
Figure 1. Algorithm for patients with suspected ACS.
Substantial variations exist between diagnostic cut-off hs-cTn values across individual laboratories, which can be attributed to biological as well as analytical variabilities. With improvements in hs-cTn analytical assays, population differences in troponin levels have also been revealed, which could confound clinical decision making [6]. However, these differences are more apparent than real due to differences in the composition of the reference population and the statistical treatment of the reference data [7].
2. Laboratory Considerations for hs-cTn
In the recent past, it has become evident that the hs-cTn 99th-percentile upper reference limit (URL) values are influenced by factors such as age [8][9][10], sex [4][11], health status [12][13], and the statistical approach used to derive the URL [14]. In light of this, reputable scientific societies such as the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) have issued specific recommendations aimed at standardizing the establishment of 99th-percentile URLs for hs-cTn [15]. These recommendations are intended to promote consistency in the interpretation and application of hs-cTn.
2.1. Analytical Designation
To be classified as a high-sensitivity troponin assay, it must meet the following two analytical criteria: (1) have a total imprecision (CV) of ≤10% at the sex-specific 99th-percentile levels, and (2) detect troponin concentrations above the assay’s limit of detection (LoD) in at least 50% of healthy individuals for both men and women [14]. Here, it would be useful to review the meaning of the limit of blank (LoB), LoD, and the limit of quantitation (LoQ) of troponin assays. The CLSI guideline EP17-A2 provides recommendations to standardize the related terminology and statistically define the detection capabilities in terms of the LoB, LoD, and LoQ [16]. The LoB refers to the highest troponin signal generated by the assay while analyzing replicates of a sample containing no analyte (zero calibrator) for the cardiac troponin assay. It is derived statistically as LoB = mean (zero calibrator) + 1.645 × SD (zero calibrator) [17]. Thus, it represents the ‘signal noise’ inherent in the analytical system. The LoD, which always exceeds the LoB, represents the lowest concentration of cardiac troponin that the assay can reliably detect with a 95% confidence level but this may not be precise enough for accurate reporting. The LoQ is the lowest cTn value that demonstrates a 20% imprecision (CV) and can be reliably reported; the LoQ is always greater than the LoD [18].
Various platforms have reported significantly lower hs-cTn values among women compared with men, which may be attributed to their lower cardiac mass [19]. A recent cardiac magnetic resonance imaging (MRI) study reported a left ventricular mass of 39 g/m2 for women and 50 g/m2 for men [20]. Thus, the use of a single diagnostic threshold for cTn values in both men and women may result in the underdiagnosis of myocardial infarction in women and overdiagnosis in men [21][22][23]. This has led to the inclusion of sex-specific 99th-percentile URLs for hs-cTn in the universal definitions of myocardial infarction [4] and in the International Federation of Clinical Chemistry (IFCC) guidelines [24][25]. However, the recent European guidelines continue to advocate for single gender-independent troponin cut-offs [26].
2.2. Defining the Reference Population
The reference cohort should comprise healthy individuals; preinclusion screening should be performed through a questionnaire survey and clinical examination to ensure that the population is healthy [27]. Certain comorbidities have been reported to substantially influence the measured hs-cTn concentrations, ultimately affecting the determination of URLs and rendering it unacceptable to include individuals with such comorbidities in the reference cohort. Subclinical myocardial injury and hemodynamic stress raise serum levels of troponin [28]. Furthermore, elevated hs-cTnT levels have been associated with increased incidence rates of cardiac failure [29]. As such, it has been recommended to exclude individuals with N terminal pro-brain natriuretic peptide (NT-proBNP) levels >125 ng/L or brain natriuretic peptide (BNP) levels >35 ng/L [30]. Diabetes mellitus and renal dysfunction are other conditions that need to be excluded. Individuals with chronic hyperglycemia exhibit reduced troponin elimination as a consequence of reduced glomerular filtration. Additionally, microvascular damage due to hyperglycemia may lead to myocardial injury and ischemia, subsequently increasing the levels of cTns in the circulation. Thus, glycosylated hemoglobin (HbA1c) screening would be useful while selecting the reference population; individuals with HbA1c values ≥48 mmol/mol (6.5%) are excluded [24][31].
Another important condition that needs to be excluded is chronic renal disease. Higher troponin concentrations have been reported in the presence of renal dysfunction [32][33], which can be partially explained by impaired renal clearance [34]; this is especially so with troponin-T compared to Troponin I [35]. Studies have also reported that troponin increases with a declining estimated glomerular filtration rate (eGFR) between 60 and 90 mL/min/1.73 m2 [36]. In a prospective study including cardio-renal healthy participants in Singapore, it was observed that for every 10 mL/min/1.73 m2 reduction in eGFR below 90 mL/min/1.73 m2, there was a significant stepwise increase in hs-cTnT concentrations, though not for hs-cTnI [20]. Thus, researchers feel that the more liberal eGFR recommendation by the expert committee guideline should be tightened to include only subjects with eGFR >90 mL/min/1.73 m2. The current IFCC guidelines recommend excluding only those with eGFR <60 mL/min/1.73 m2 [24].
Of note, several studies [37][38] have highlighted the influence of the reference population selection strategy on the hs-cTn 99th-percentile URLs. Reference cohorts that rigorously exclude individuals taking medications associated with cardiovascular disease or risk factors (e.g., antihypertensive, antidiabetes, or lipid-lowering drugs) tend to exhibit lower URLs compared to populations with less stringent screening criteria. The Association for Diagnostics and Laboratory Medicine (ADLM; formerly AACC) [39] also recommends the exclusion of those with cancer and thyroid diseases. Individuals with an abnormal body mass index (BMI) may also need to be excluded as obesity has been found to be independently associated with elevated hs-cTn levels [40].
2.3. Derivation of the 99th Percentile
The reference population should ideally consist of approximately equal proportions of 400 males and females each [24][39], spanning an age range from 18 to 80 years, with all relevant ethnic and racial groups adequately represented. As opposed to the earlier recommendation of including a minimum of 300 men and 300 women, the recent report of the IFCC Task Force on Clinical Applications of Cardiac Biomarkers (IFCC TF-CB) now recommends a minimum of 800 total subjects (400 men and 400 women) to be included in the reference cohort [24]. A sample size of 300 subjects per gender was sufficient to generate a URL with only a 90% confidence interval (CI) ±10% while a sample size of 400 subjects per gender provides a URL with a 95% CI ± 10%.
It should be noted that the sample size requirement is also influenced by the statistical method used to calculate the URL. While various statistical methods can be employed to establish reference limits, the Clinical and Laboratory Standards Institute (CLSI) suggests using a one-tailed nonparametric method [41]. Given the meticulous selection of participants in the healthy cohort, the risk of outliers arising from undetected subclinical comorbidities should be minimized. Therefore, the process of excluding outliers should also be thorough. The Reed/Dixon criteria is a preferable approach by some labs, as it tends to exclude fewer subjects compared to the Tukey method [42]; researchers favor the more stringent Tukey approach.
The 99th-percentile URL is a key metric that is meticulously derived since the URL is used to minimize any statistical uncertainty. It is important to note that the 99th-percentile URL is not a magic number but simply a value that is used to define the upper limit of the normal range for hs-cTn in the reference population. Values above the URL are considered to be abnormal and may indicate the presence of myocardial injury or infarction; the higher the value, the greater the likelihood is of AMI. However, for interpreting hs-cTn results, it is important to consider some analytical factors as well as clinical conditions that may lead to altered troponin levels. Besides MI, there are many other non-MI clinical entities associated with elevated troponins that should be recognized while interpreting hs-cTn results (Table 1). These conditions may be due to impaired oxygen supply and demand as well as a complex mix of comorbid conditions acting in concert [26].
Table 1. Non-MI causes of elevated troponins.
| Cardiac Conditions |
|---|
| Congestive cardiac failure (acute and chronic) [29] |
| Atrial fibrillation [43] |
| Stable coronary artery disease [28] |
| Pericarditis/myocarditis [44] |
| Aortic dissection [45] |
| Cardiotoxic chemotherapy [46] |
| Non-cardiac conditions |
| Chronic kidney disease [32] |
| Diabetes [32] |
| Sepsis [47] |
| Cancer [24] |
| Untreated thyroid disorders [39] |
| Strenuous exercise [48][49] |
| Presence of cTn autoantibodies [50] |
| Myopathies [50] |
| Ischemic stroke [51] |
2.4. Quality Control (QC) Recommendations
With the evolution of hs-cTn assays over the recent years and the pivotal role assumed by troponins in the diagnosis of AMI, the analytical performance criteria have become more rigorous for hs-cTn assays. It is of utmost importance to monitor assay performance across a range of concentrations that are relevant for clinical decision making, including the 99th-percentile URL. Thus, recent guidelines [52] have recommended the use of three levels of QC materials for hs-cTn assays, as follows:
-
Level 1 QC: This concentration should fall within the range between the LoD and the lowest sex-specific 99th percentile. It is important to evaluate the lower end of the assay’s analytical sensitivity and precision, since low cTn levels (<LoD) are used to rule out AMI/myocardial injury.
-
Level 2 QC: This concentration should be higher than but close to (within 20% of) the highest sex-specific 99th-percentile URL. It allows for the assessment of the assay’s accuracy and precision near the URL.
-
Level 3 QC: This concentration should be significantly elevated to challenge the upper analytical range of reportable cTn results. It involves using concentrations that are multiples above the 99th-percentile concentration. This is important to assess the reproducibility of the assay at high cTn concentrations.
2.5. Reporting Units for hs-cTn
Besides the QC recommendations, the consensus among prominent specialist lab organizations like the IFCC and the ADLM is that hs-cTn values should be expressed in nanograms per liter (ng/L) and reported as whole numbers [24][39]. This avoids confusion and potential transcription errors, especially when comparing results with non-high-sensitivity assays. This scenario occurs when the Emergency Department deploys point-of-care (POC) troponin testing (conventional cTn) while the central laboratory uses a hs-cTn method. In addition, adopting this reporting convention helps avoid errors in data recording and ensures clarity and consistency in the reporting of hs-cTn results.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics14010087
References
- Ministry of Health, Singapore. Principal Causes of Death. Available online: https://www.moh.gov.sg/resources-statistics/singapore-health-facts/principal-causes-of-death (accessed on 9 October 2023).
- Wu, J.; Gale, C.P.; Hall, M.; Dondo, T.B.; Metcalfe, E.; Oliver, G.; Batin, P.D.; Hemingway, H.; Timmis, A.; West, R.M. Impact of initial hospital diagnosis on mortality for acute myocardial infarction: A national cohort study. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 139–148.
- Park, K.C.; Gaze, D.C.; Collinson, P.O.; Marber, M.S. Cardiac troponins: From myocardial infarction to chronic disease. Cardiovasc. Res. 2017, 113, 1708–1718.
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). J. Am. Coll. Cardiol. 2018, 72, 2231–2264.
- Krychtiuk, K.A.; Newby, L.K. High-Sensitivity Cardiac Troponin Assays: Ready for Prime Time! Annu. Rev. Med. 2024, 75, 9.1–9.16.
- Kimenai, D.M.; Janssen, E.B.N.J.; Eggers, K.M.; Lindahl, B.; den Ruijter, H.M.; Bekers, O.; Appelman, Y.; Meex, S.J.R. Sex-Specific Versus Overall Clinical Decision Limits for Cardiac Troponin I and T for the Diagnosis of Acute Myocardial Infarction: A Systematic Review. Clin. Chem. 2018, 64, 1034–1043.
- Lee, D.J.W.; Aw, T.C. Population differences and high-sensitivity troponin values: A narrative review. J. Lab. Precis. Med. 2023, 8, 14.
- Hickman, P.E.; Abhayaratna, W.P.; Potter, J.M.; Koerbin, G. Age-related differences in hs-cTnI concentration in healthy adults. Clin. Biochem. 2019, 69, 26–29.
- Clerico, A.; Masotti, S.; Musetti, V.; Ripoli, A.; Aloe, R.; Di Pietro, M.; Rizzardi, S.; Dittadi, R.; Carrozza, C.; Belloni, L.; et al. Evaluation of 99th percentile and reference change values of the hs-cTnI method using ADVIA Centaur XPT platform: A multicenter study. Clin. Chim. Acta 2019, 495, 161–166.
- Agnello, L.; Bellia, C.; Scazzone, C.; Bivona, G.; Iacolino, G.; Gambino, C.M.; Muratore, M.; Lo Sasso, B.; Ciaccio, M. Establishing the 99th percentile for high sensitivity cardiac troponin I in healthy blood donors from Southern Italy. Biochem. Med. 2019, 29, 020901.
- Aw, T.C.; Phua, S.K.; Tan, S.P. Measurement of cardiac troponin I in serum with a new high-sensitivity assay in a large multi-ethnic Asian cohort and the impact of gender. Clin. Chim. Acta 2013, 422, 26.
- Sedighi, S.M.; Prud’Homme, P.; Ghachem, A.; Lepage, S.; Nguyen, M.; Fulop, T.; Khalil, A. Increased level of high-sensitivity cardiac Troponin T in a geriatric population is determined by comorbidities compared to age. Int. J. Cardiol. Heart Vasc. 2019, 8, 187–191.
- Odsæter, I.H.; Grenne, B.; Hov, G.G.; Laugsand, L.E.; Wiseth, R.; Mikkelsen, G. Establishing the 99th percentile of a novel assay for high-sensitivity troponin I in a healthy blood donor population. Clin. Chem. Lab. Med. 2020, 58, 1557–1563.
- Eggers, K.M.; Apple, F.S.; Lind, L.; Lindahl, B. The applied statistical approach highly influences the 99th percentile of cardiac troponin I. Clin. Biochem. 2016, 49, 1109–1112.
- Apple, F.S.; Collinson, P.O. IFCC Task Force on Clinical Applications of Cardiac Biomarkers, Analytical Characteristics of High-Sensitivity Cardiac Troponin Assays. Clin. Chem. 2012, 58, 54–61.
- Clinical Laboratory Standards Institute (CLSI). Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures, 2nd ed.; CLSI Guideline EP17-A2; CLSI: Wayne, PA, USA, 2012.
- Armbruster, D.A.; Pry, T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008, 1, 49–52.
- Apple, F.S.; Sandoval, Y.; Jaffe, A.S.; Ordonez-Llanos, J. IFCC Task Force on Clinical Applications of Cardiac Bio-Markers, Cardiac Troponin Assays: Guide to Understanding Analytical Characteristics and Their Impact on Clinical Care. Clin. Chem. 2017, 63, 73–81.
- Solola Nussbaum, S.; Henry, S.; Yong, C.M.; Daugherty, S.L.; Mehran, R.; Poppas, A. Sex-Specific Considerations in the Presentation, Diagnosis, and Management of Ischemic Heart Disease. J. Am. Coll. Cardiol. 2022, 79, 1398–1406.
- Aw, T.C.; Huang, W.T.; Le, T.T.; Pua, C.J.; Ang, B.; Phua, S.K.; Yeo, K.K.; Cook, S.A.; Chin, C.W.L. High-Sensitivity cardiac Troponins in Cardio-Healthy Subjects: A Cardiovascular Magnetic Resonance Imaging Study. Sci. Rep. 2018, 8, 15409.
- Chaulin, A.M. Gender Specificities of Cardiac Troponin Serum Levels: From Formation Mechanisms to the Diagnostic Role in Case of Acute Coronary Syndrome. Life 2023, 13, 267.
- Mueller, T.; Egger, M.; Peer, E.; Jani, E.; Dieplinger, B. Evaluation of sex-specific cut-off values of high-sensitivity cardiac troponin I and T assays in an emergency department setting—Results from the Linz Troponin (LITROP) study. Clin. Chim. Acta 2018, 487, 66–74.
- Lee, K.K.; Ferry, A.V.; Anand, A.; Strachan, F.E.; Chapman, A.R.; Kimenai, D.M.; Meex, S.J.R.; Berry, C.; Findlay, I.; Reid, A.; et al. High-STEACS Investigators. Sex-Specific Thresholds of High-Sensitivity Troponin in Patients with Suspected Acute Coronary Syndrome. J. Am. Coll. Cardiol. 2019, 74, 2032–2043.
- Aakre, K.M.; Saenger, A.K.; Body, R.; Collinson, P.; Hammarsten, O.; Jaffe, A.S.; Kavsak, P.; Omland, T.; Ordonez-Lianos, J.; Apple, F.S. Analytical Considerations in Deriving 99th Percentile Upper Reference Limits for High-Sensitivity Cardiac Troponin Assays: Educational Recommendations from the IFCC Committee on Clinical Application of Cardiac Bio-Markers. Clin. Chem. 2022, 68, 1022–1030.
- Sandoval, Y.; Apple, F.S.; Mahler, S.A.; Body, R.; Collinson, P.O.; Jaffe, A.S. International Federation of Clinical Chemistry and Laboratory Medicine Committee on the Clinical Application of Cardiac Biomarkers. High-Sensitivity Cardiac Troponin and the 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guidelines for the Evaluation and Diagnosis of Acute Chest Pain. Circulation 2022, 146, 569–581.
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC guidelines for the management of acute coronary syndromes: Developed by the task force of the European Societry of Cardiology. Eur. Heart J. 2023, 44, 3720–3826.
- Zhang, X.; Han, X.; Zhao, M.; Mu, R.; Wang, S.; Yun, K.; Shang, H. Determination of high-sensitivity cardiac troponin T upper reference limits under the improved selection criteria in a Chinese population. J. Clin. Lab. Anal. 2020, 34, e23007.
- Shemisa, K.; Bhatt, A.; Cheeran, D.; Neeland, I.J. Novel Biomarkers of Subclinical Cardiac Dysfunction in the General Population. Curr. Heart Fail. Rep. 2017, 14, 301–310.
- McEvoy, J.W.; Chen, Y.; Ndumele, C.E.; Solomon, S.D.; Nambi, V.; Ballantyne, C.M.; Blumenthal, R.S.; Coresh, J.; Selvin, E. Six-Year Change in High-Sensitivity Cardiac Troponin T and Risk of Subsequent Coronary Heart Disease, Heart Failure, and Death. JAMA Cardiol. 2016, 1, 519–528.
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380.
- Šimić, S.; Svaguša, T.; Prkačin, I.; Bulum, T. Relationship between hemoglobin A1c and serum troponin in patients with diabetes and cardiovascular events. J. Diabetes Metab. Disord. 2019, 18, 693–704.
- Cardinaels, E.P.; Altintas, S.; Versteylen, M.O.; Joosen, I.A.; Jellema, L.J.; Wildberger, J.E.; Das, M.; Crijns, H.J.; Bekers, O.; van Dieijen-Visser, M.P.; et al. High-Sensitivity Cardiac Troponin Concentrations in Patients with Chest Discomfort: Is It the Heart or the Kidneys As Well? PLoS ONE 2016, 11, e0153300.
- Dubin, R.F.; Li, Y.; He, J.; Jaar, B.G.; Kallem, R.; Lash, J.P.; Makos, G.; Rosas, S.E.; Soliman, E.Z.; Townsend, R.R.; et al. CRIC Study Investigators. Predictors of high sensitivity cardiac troponin T in chronic kidney disease patients: A cross-sectional study in the chronic renal insufficiency cohort (CRIC). BMC Nephrol. 2013, 22, 229.
- Muslimovic, A.; Fridén, V.; Tenstad, O.; Starnberg, K.; Nyström, S.; Wesén, E.; Esbjörner, E.K.; Granholm, K.; Lindahl, B.; Hammarsten, O. The Liver and Kidneys mediate clearance of cardiac troponin in the rat. Sci. Rep. 2020, 10, 6791.
- Ledwoch, J.; Krauth, A.; Kraxenberger, J.; Schneider, A.; Leidgschwendner, K.; Schneider, V.; Müller, A.; Laugwitz, K.L.; Kupatt, C.; Martens, E. Accuracy of high-sensitive troponin depending on renal function for clinical outcome prediction in patients with acute heart failure. Heart Vessels 2022, 37, 69–76.
- Martens, R.J.; Kimenai, D.M.; Kooman, J.P.; Stehouwer, C.D.; Tan, F.E.; Bekers, O.; Dagnelie, P.C.; van der Kallen, C.J.; Kroon, A.A.; Leunissen, K.M.; et al. Estimated Glomerular Filtration Rate and Albuminuria Are Associated with Biomarkers of Cardiac Injury in a Population-Based Cohort Study: The Maastricht Study. Clin. Chem. 2017, 63, 887–897.
- Collinson, P.O.; Heung, Y.M.; Gaze, D.; Boa, F.; Senior, R.; Christenson, R.; Apple, F.S. Influence of population selection on the 99th percentile reference value for cardiac troponin assays. Clin. Chem. 2012, 58, 219–225.
- Koerbin, G.; Abhayaratna, W.P.; Potter, J.M.; Apple, F.S.; Jaffe, A.S.; Ravalico, T.H.; Hickman, P.E. Effect of population selection on 99th percentile values for a high sensitivity cardiac troponin I and T assays. Clin. Biochem. 2013, 46, 1636–1643.
- Saenger, A. High-Sensitivity Cardiac Troponin. Pearls of Laboratory Medicine. Trainee Council in English. 20 August 2021. Available online: https://www.aacc.org/science-and-research/clinical-chemistry-trainee-council/trainee-council-in-english/pearls-of-laboratory-medicine/2021/high-sensitivity-cardiac-troponin (accessed on 9 October 2023).
- Huang, J.; Liu, M.; Su, E.; Yu, P.; Jiang, H.; Zhao, J.; Ge, J. Elevated circulating high-sensitivity cardiac troponin t and cardiac remodeling in obesity. BMC Cardiovasc. Disord. 2021, 21, 620.
- Clinical Laboratory Standards Institute (CLSI). Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory, 3rd ed.; CLSI Guideline EP28-A3c; CLSI: Wayne, PA, USA, 2016.
- Ahn, S.; Kim, H.K.; Lee, W.; Chun, S.; Min, W.K. Effect of Outlier Elimination on the 99th Percentile Upper Reference Limits of High-Sensitivity Cardiac Troponin I Assays Based on a Strictly Selected Healthy Reference Population. Ann. Lab. Med. 2022, 42, 331–341.
- Sepehri Shamloo, A.; Arya, A.; Darma, A.; Nedios, S.; Döring, M.; Bollmann, A.; Dagres, N.; Hindricks, G. Atrial fibrillation: Is there a role for cardiac troponin? Diagnosis 2020, 8, 295–303.
- Janardhanan, R. Myocarditis with very high troponins: Risk stratification by cardiac magnetic resonance. J. Thorac. Dis. 2016, 8, E1333–E1336.
- Liu, S.; Song, C.; Bian, X.; Wang, H.; Fu, R.; Zhang, R.; Yuan, S.; Dou, K. Elevated cardiac troponin I and short-term mortality in patients with acute type A aortic dissection. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 597–606.
- Pudil, R.; Mueller, C.; Čelutkienė, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983.
- Matsunaga, N.; Yoshioka, Y.; Fukuta, Y. Extremely high troponin levels induced by septic shock: A case report. J. Med. Case Reports. 2021, 15, 466.
- Airaksinen, K.E.J. Cardiac Troponin Release After Endurance Exercise: Still Much to Learn. J. Am. Heart Assoc. 2020, 9, e015912.
- Aw, T.C.; van Wijk, X.M.; Wu, A.H.; Jaffe, A.S. Release of cardiac troponin using a high sensitivity assay after exercise: Type 2 acute myocardial infarction? Clin. Chim. Acta 2015, 446, 6–8.
- Eggers, K.M.; Hammarsten, O.; Lindahl, B. Differences between high-sensitivity cardiac troponin T and I in stable populations: Underlying causes and clinical implications. Clin. Chem. Lab. Med. 2022, 61, 380–387.
- Nam, K.W.; Kim, C.K.; Yu, S.; Chung, J.W.; Bang, O.Y.; Kim, G.M.; Jung, J.M.; Song, T.J.; Kim, Y.J.; Kim, B.J.; et al. Elevated troponin levels are associated with early neurological worsening in ischemic stroke with atrial fibrillation. Sci. Rep. 2020, 10, 12626.
- Wu, H.B.; Christenson, R.H.; Greene, D.N.; Jaffe, A.S.; Kavsak, P.A.; Ordonez-Llanos, J.; Apple, F.S. Clinical Laboratory Practice Recommendations for the Use of Cardiac Troponin in Acute Coronary Syndrome: Expert Opinion from the Academy of the American Association for Clinical Chemistry and the Task Force on Clinical Applications of Cardiac Bio-Markers of the International Federation of Clinical Chemistry and Laboratory Medicine. Clin. Chem. 2018, 64, 645–655.
This entry is offline, you can click here to edit this entry!
