Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cardiac & Cardiovascular Systems
High-sensitivity cardiac troponin (hs-cTn) assays have revolutionized the assessment of myocardial stress and injury due to their superior sensitivity and accuracy in detecting even minor cardiac damage. However, hs-cTn is often included in the initial blood draw for baseline assessment in such patients and has prognostic value in predicting in-hospital and long-term mortality.
- acute myocardial infarction
- cardiac biomarkers
- high-sensitivity cardiac troponins
1. Introduction
Cardiovascular diseases are the leading cause of death globally. In Singapore, ischemic heart diseases (IHDs) accounted for 19.7% of total deaths in 2022 [1]. A significant number of patients with acute coronary syndrome (ACS) often fail to receive timely treatment owing to incorrect preliminary diagnosis [2]. Most cases of acute myocardial infarction (AMI) present with chest pain, and a proper, rapid diagnostic assessment is essential so that early and effective treatment can be implemented. Similarly, prompt the rule-out of an AMI would avoid unnecessary hospital admissions.
In the initial evaluation of suspected ACS clinical assessment, 12-lead electrocardiogram (ECG), and cardiac troponins (cTn) play a crucial role [3,4]. ST-elevation MI (STEMI) requires immediate reperfusion therapy while very high-risk non-STEMI needs immediate invasive strategy (angiography ± percutaneous coronary intervention). For non-STEMI with high-risk features, angiography within 24 h can be considered. This algorithm is illustrated in Figure 1. Over the recent years, high-sensitivity cardiac troponin (hs-cTn) assays have revolutionized the assessment of myocardial stress and injury due to their superior sensitivity and accuracy in detecting even minor cardiac damage [5]. Patients with STEMI or very high-risk non-STEMI do not need troponin for diagnosis, but their management should not be delayed by waiting for troponin results to be available. However, hs-cTn is often included in the initial blood draw for baseline assessment in such patients and has prognostic value in predicting in-hospital and long-term mortality. For the remining patients with suspected ACS, hs-cTn is pivotal for confirmation of diagnosis, risk assessment/prognostication and subsequent management.
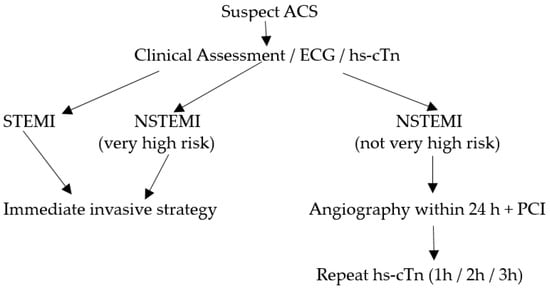
Figure 1. Algorithm for patients with suspected ACS.
Substantial variations exist between diagnostic cut-off hs-cTn values across individual laboratories, which can be attributed to biological as well as analytical variabilities. With improvements in hs-cTn analytical assays, population differences in troponin levels have also been revealed, which could confound clinical decision making [6]. However, these differences are more apparent than real due to differences in the composition of the reference population and the statistical treatment of the reference data [7].
2. Laboratory Considerations for hs-cTn
In the recent past, it has become evident that the hs-cTn 99th-percentile upper reference limit (URL) values are influenced by factors such as age [8,9,10], sex [4,11], health status [12,13], and the statistical approach used to derive the URL [14]. In light of this, reputable scientific societies such as the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) have issued specific recommendations aimed at standardizing the establishment of 99th-percentile URLs for hs-cTn [15]. These recommendations are intended to promote consistency in the interpretation and application of hs-cTn.
2.1. Analytical Designation
To be classified as a high-sensitivity troponin assay, it must meet the following two analytical criteria: (1) have a total imprecision (CV) of ≤10% at the sex-specific 99th-percentile levels, and (2) detect troponin concentrations above the assay’s limit of detection (LoD) in at least 50% of healthy individuals for both men and women [14]. Here, it would be useful to review the meaning of the limit of blank (LoB), LoD, and the limit of quantitation (LoQ) of troponin assays. The CLSI guideline EP17-A2 provides recommendations to standardize the related terminology and statistically define the detection capabilities in terms of the LoB, LoD, and LoQ [16]. The LoB refers to the highest troponin signal generated by the assay while analyzing replicates of a sample containing no analyte (zero calibrator) for the cardiac troponin assay. It is derived statistically as LoB = mean (zero calibrator) + 1.645 × SD (zero calibrator) [17]. Thus, it represents the ‘signal noise’ inherent in the analytical system. The LoD, which always exceeds the LoB, represents the lowest concentration of cardiac troponin that the assay can reliably detect with a 95% confidence level but this may not be precise enough for accurate reporting. The LoQ is the lowest cTn value that demonstrates a 20% imprecision (CV) and can be reliably reported; the LoQ is always greater than the LoD [18].
Various platforms have reported significantly lower hs-cTn values among women compared with men, which may be attributed to their lower cardiac mass [19]. A recent cardiac magnetic resonance imaging (MRI) study reported a left ventricular mass of 39 g/m2 for women and 50 g/m2 for men [20]. Thus, the use of a single diagnostic threshold for cTn values in both men and women may result in the underdiagnosis of myocardial infarction in women and overdiagnosis in men [21,22,23]. This has led to the inclusion of sex-specific 99th-percentile URLs for hs-cTn in the universal definitions of myocardial infarction [4] and in the International Federation of Clinical Chemistry (IFCC) guidelines [24,25]. However, the recent European guidelines continue to advocate for single gender-independent troponin cut-offs [26].
2.2. Defining the Reference Population
The reference cohort should comprise healthy individuals; preinclusion screening should be performed through a questionnaire survey and clinical examination to ensure that the population is healthy [27]. Certain comorbidities have been reported to substantially influence the measured hs-cTn concentrations, ultimately affecting the determination of URLs and rendering it unacceptable to include individuals with such comorbidities in the reference cohort. Subclinical myocardial injury and hemodynamic stress raise serum levels of troponin [28]. Furthermore, elevated hs-cTnT levels have been associated with increased incidence rates of cardiac failure [29]. As such, it has been recommended to exclude individuals with N terminal pro-brain natriuretic peptide (NT-proBNP) levels >125 ng/L or brain natriuretic peptide (BNP) levels >35 ng/L [30]. Diabetes mellitus and renal dysfunction are other conditions that need to be excluded. Individuals with chronic hyperglycemia exhibit reduced troponin elimination as a consequence of reduced glomerular filtration. Additionally, microvascular damage due to hyperglycemia may lead to myocardial injury and ischemia, subsequently increasing the levels of cTns in the circulation. Thus, glycosylated hemoglobin (HbA1c) screening would be useful while selecting the reference population; individuals with HbA1c values ≥48 mmol/mol (6.5%) are excluded [24,31].
Another important condition that needs to be excluded is chronic renal disease. Higher troponin concentrations have been reported in the presence of renal dysfunction [32,33], which can be partially explained by impaired renal clearance [34]; this is especially so with troponin-T compared to Troponin I [35]. Studies have also reported that troponin increases with a declining estimated glomerular filtration rate (eGFR) between 60 and 90 mL/min/1.73 m2 [36]. In a prospective study including cardio-renal healthy participants in Singapore, it was observed that for every 10 mL/min/1.73 m2 reduction in eGFR below 90 mL/min/1.73 m2, there was a significant stepwise increase in hs-cTnT concentrations, though not for hs-cTnI [20]. Thus, researchers feel that the more liberal eGFR recommendation by the expert committee guideline should be tightened to include only subjects with eGFR >90 mL/min/1.73 m2. The current IFCC guidelines recommend excluding only those with eGFR <60 mL/min/1.73 m2 [24].
Of note, several studies [37,38] have highlighted the influence of the reference population selection strategy on the hs-cTn 99th-percentile URLs. Reference cohorts that rigorously exclude individuals taking medications associated with cardiovascular disease or risk factors (e.g., antihypertensive, antidiabetes, or lipid-lowering drugs) tend to exhibit lower URLs compared to populations with less stringent screening criteria. The Association for Diagnostics and Laboratory Medicine (ADLM; formerly AACC) [39] also recommends the exclusion of those with cancer and thyroid diseases. Individuals with an abnormal body mass index (BMI) may also need to be excluded as obesity has been found to be independently associated with elevated hs-cTn levels [40].
2.3. Derivation of the 99th Percentile
The reference population should ideally consist of approximately equal proportions of 400 males and females each [24,39], spanning an age range from 18 to 80 years, with all relevant ethnic and racial groups adequately represented. As opposed to the earlier recommendation of including a minimum of 300 men and 300 women, the recent report of the IFCC Task Force on Clinical Applications of Cardiac Biomarkers (IFCC TF-CB) now recommends a minimum of 800 total subjects (400 men and 400 women) to be included in the reference cohort [24]. A sample size of 300 subjects per gender was sufficient to generate a URL with only a 90% confidence interval (CI) ±10% while a sample size of 400 subjects per gender provides a URL with a 95% CI ± 10%.
It should be noted that the sample size requirement is also influenced by the statistical method used to calculate the URL. While various statistical methods can be employed to establish reference limits, the Clinical and Laboratory Standards Institute (CLSI) suggests using a one-tailed nonparametric method [41]. Given the meticulous selection of participants in the healthy cohort, the risk of outliers arising from undetected subclinical comorbidities should be minimized. Therefore, the process of excluding outliers should also be thorough. The Reed/Dixon criteria is a preferable approach by some labs, as it tends to exclude fewer subjects compared to the Tukey method [42]; researchers favor the more stringent Tukey approach.
The 99th-percentile URL is a key metric that is meticulously derived since the URL is used to minimize any statistical uncertainty. It is important to note that the 99th-percentile URL is not a magic number but simply a value that is used to define the upper limit of the normal range for hs-cTn in the reference population. Values above the URL are considered to be abnormal and may indicate the presence of myocardial injury or infarction; the higher the value, the greater the likelihood is of AMI. However, for interpreting hs-cTn results, it is important to consider some analytical factors as well as clinical conditions that may lead to altered troponin levels. Besides MI, there are many other non-MI clinical entities associated with elevated troponins that should be recognized while interpreting hs-cTn results (Table 1). These conditions may be due to impaired oxygen supply and demand as well as a complex mix of comorbid conditions acting in concert [26].
Table 1. Non-MI causes of elevated troponins.
| Cardiac Conditions |
|---|
| Congestive cardiac failure (acute and chronic) [29] |
| Atrial fibrillation [43] |
| Stable coronary artery disease [28] |
| Pericarditis/myocarditis [44] |
| Aortic dissection [45] |
| Cardiotoxic chemotherapy [46] |
| Non-cardiac conditions |
| Chronic kidney disease [32] |
| Diabetes [32] |
| Sepsis [47] |
| Cancer [24] |
| Untreated thyroid disorders [39] |
| Strenuous exercise [48,49] |
| Presence of cTn autoantibodies [50] |
| Myopathies [50] |
| Ischemic stroke [51] |
2.4. Quality Control (QC) Recommendations
With the evolution of hs-cTn assays over the recent years and the pivotal role assumed by troponins in the diagnosis of AMI, the analytical performance criteria have become more rigorous for hs-cTn assays. It is of utmost importance to monitor assay performance across a range of concentrations that are relevant for clinical decision making, including the 99th-percentile URL. Thus, recent guidelines [52] have recommended the use of three levels of QC materials for hs-cTn assays, as follows:
-
Level 1 QC: This concentration should fall within the range between the LoD and the lowest sex-specific 99th percentile. It is important to evaluate the lower end of the assay’s analytical sensitivity and precision, since low cTn levels (<LoD) are used to rule out AMI/myocardial injury.
-
Level 2 QC: This concentration should be higher than but close to (within 20% of) the highest sex-specific 99th-percentile URL. It allows for the assessment of the assay’s accuracy and precision near the URL.
-
Level 3 QC: This concentration should be significantly elevated to challenge the upper analytical range of reportable cTn results. It involves using concentrations that are multiples above the 99th-percentile concentration. This is important to assess the reproducibility of the assay at high cTn concentrations.
2.5. Reporting Units for hs-cTn
Besides the QC recommendations, the consensus among prominent specialist lab organizations like the IFCC and the ADLM is that hs-cTn values should be expressed in nanograms per liter (ng/L) and reported as whole numbers [24,39]. This avoids confusion and potential transcription errors, especially when comparing results with non-high-sensitivity assays. This scenario occurs when the Emergency Department deploys point-of-care (POC) troponin testing (conventional cTn) while the central laboratory uses a hs-cTn method. In addition, adopting this reporting convention helps avoid errors in data recording and ensures clarity and consistency in the reporting of hs-cTn results.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics14010087
This entry is offline, you can click here to edit this entry!
