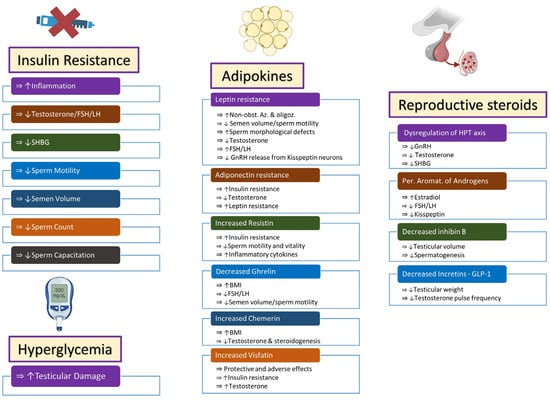
Insulin resistance, hyperglycemia, and adipokines have been implicated as causes of male infertility in obese males [
14,
15,
16,
17,
18,
19] (
Figure 1).
Figure 1. Endocrine changes in obese males lead to infertility via multiple pathways. Increased insulin resistance and hyperglycemia affect fertility via effects on inflammatory molecules, steroidogenesis, and semen quality. Adipokines behave like endocrine organs and secrete multiple hormones that have protective or adverse effects on semen and sperm quality, steroidogenesis, insulin resistance, and inflammatory mechanisms. Dysregulation of steroidogenesis occurs and leads to alterations in steroidogenesis through central and peripheral pathways. Symbols: (⇒): is associated with/is correlated with; (↑): increased; (↓): decreased. Abbreviations: FSH: follicle-stimulating hormone; LH: luteinizing hormone; SHBG: sex hormone-binding globulin; Non-obst. Az: non-obstructive azoospermia; Oligoz: oligozoospermia; GnRH: gonadotropin-releasing hormone; BMI: body mass index; HPT axis: hypothalamus–pituitary–testicular axis; Per. Aromat.: peripheral aromatization; GLP-1: glucagon-like peptide-1.
2. Insulin Resistance and Hyperglycemia
2.1. Insulin Resistance
The term “metabolic syndrome” encompasses a range of related conditions, including diabetes mellitus, hypertension, dyslipidemia, coronary artery disease, and obesity [
19]. Insulin resistance is the inability of a known dose of exogenous or endogenous insulin to stimulate glucose uptake and metabolism in an individual to the same extent that it does in the general population in good health [
20,
21]. Increased insulin resistance has been noted in males with unexplained infertility [
22].
Insulin resistance increases inflammation, lowers male sex hormone release, lowers sex hormone binding globulin levels, and aggravates obesity [
16,
20,
23,
24]. Insulin resistance has been shown to independently influence sperm motility and, when associated with obesity, decrease semen volume and sperm count [
19,
21,
25].
Freshly ejaculated sperm are still without progressive motility or the capacity to fertilize. They first need to go through a process called capacitation, which entails a progression of biochemical and physiological alterations. A key requirement for fertilization is capacitation [
26,
27]. Insulin has been found to be secreted by sperm. It has been shown that there is a significant difference between insulin release in capacitated and non-capacitated sperm, pointing to a possible active role for insulin in capacitation. Insulin resistance could interfere with the distal effects of autocrine insulin secretion from the sperm and negatively affect capacitation [
28].
Extra-pancreatic insulin receptors have been demonstrated to be present in sperm cells in mammals. The activation of these receptors has been shown to enhance sperm motility. It is also noted that the male germ cell line is capable of producing insulin itself [
29,
30,
31]. Whether insulin resistance seen in obesity affects these receptors is not known.
2.2. Hyperglycemia
Hyperglycemia has been shown to negatively affect male fertility, both in Type 1 and Type 2 diabetes mellitus [
32,
33,
34]. Hyperglycemia has been demonstrated to induce testicular damage via diacylglycerol–protein kinase C and the polyol pathways in the Type 1 diabetes mellitus mouse model [
35]. Advanced glycation end-products (AGE) are the result of non-enzymatic glycosylation pathways [
36]. AGE receptors are expressed throughout testicular tissue. The excess binding of AGE to these receptors is postulated to play a role in the development of infertility. Hyperglycemia is also shown to affect fertility by stimulating the inflammatory pathways and increasing the production of interleukin-1 and tumor necrosis factor-α [
24,
32,
33,
35,
36].
3. Adipokines
Up until the 1980s, adipose tissue was thought to be an inert reservoir of energy stored as triglycerides [
5]. However, adipose tissue is now understood to be a significant endocrine organ that produces several peptide hormones called adipokines, such as leptin, adiponectin, resistin, and many others [
5,
37,
38]. Other chemicals secreted by adipose tissue include molecules such as cytokines and chemokines [
5].
Adipokines regulate a variety of physiologic functions, including hunger, metabolism, cardiovascular function, immunity, and reproduction [
14]. Adipokines like leptin, adiponectin, resistin, ghrelin, orexin, and obestatin interfere with normal reproductive hormonal regulation in obese male adults [
11]. It has been theorized that adipokines are significantly implicated in the pathophysiology of poor reproductive outcomes in these individuals, directly or indirectly [
14]. However, some studies have failed to show any major effect of adipokines on peripheral sites in the male reproductive system [
15]. The expression of various steroidogenic genes is directly impacted by adipokines [
37].
3.1. Leptin
Leptin is a non-glycosylated peptide that is largely secreted by adipocytes [
13]. It is a key regulatory adipokine that plays a fundamental role in a variety of metabolic processes, including reproductive performance [
11,
39].
Childhood obesity has been reported in families with congenital leptin deficiency [
37]. Leptin has powerful anorexigenic effects, decreasing appetite by inhibiting the orexigenic factors neuropeptide Y and agouti-related peptide and augmenting the effects of the anorexigenic peptides alpha-MSH/pro-opiomelanocortin and cocaine- and amphetamine-related transcript (CART). It also raises calorie expenditure. The Janus kinase/signal transducer and activator of the transcription complex is activated by leptin, which, in turn, affects sex hormone expression [
38].
Numerous studies have reported a significant correlation between the levels of leptin, obesity, and infertility with respect to the hypothalamic–pituitary–testicular axis, the regulation of androgen levels, and the production of sperm. Elevated levels of leptin have been observed in obese infertile male individuals with disorders affecting the parenchyma of the testicles, such as nonobstructive azoospermia and oligozoospermia.
Furthermore, elevated serum leptin levels are associated with decreases in serum testosterone and sperm parameters and increases in follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels, and it can cause abnormal sperm morphology [
11,
13,
15,
37,
40].
Leptin operates in a narrow range of optimum functions. Excess or deficits in the level of leptins adversely affect reproductive functions. Leptin acts directly on the kisspeptin neurons in the hypothalamus to stimulate the release of gonadotropin-releasing hormone (GnRH). Poor reproductive status, which is associated with increasing obesity, is postulated to be due to the effect of leptin on the kisspeptin-GnRH pathway [
39,
41].
Excessive levels of leptins are paradoxically found in obese adults [
24]. A leptin resistance pathway has been hypothesized, similar to the insulin resistance pathway, whereby the excessive level of leptin has an inhibitory effect on the kisspeptin neurons, the function of the testes, and spermatogenesis [
11,
24,
38,
41].
Elevated levels of leptin also seem to weaken the nutritional function of Sertoli cells in spermatogenesis. Hyperleptinemia can impair testosterone production in the Leydig cells. It is also associated with mitochondrial dysfunction, increasing oxidative stress and affecting spermatozoal function [
5].
It is probable that measuring leptin and FSH levels is a sensitive biomarker in predicting the successful retrieval of sperm in obese adult males with non-obstructive azoospermia [
38].
3.2. Adiponectin
The most abundant adipose-tissue-secreted hormone is adiponectin, which plays important functions in glucose regulation and lipid metabolism. Adiponectin has two distinctive receptors, namely, AdipoR1, which is universally expressed in skeletal muscle, and AdipoR2, which is principally expressed in the liver and adipose tissue. Both receptors are also expressed by Leydig cells, spermatozoa, and the epididymis. Adiponectin shields Leydig cells from the injurious effects of cytokines by inhibiting nuclear factor-KB signaling through the stimulation of testicular 5′ AMP-activated protein kinase [
38].
The production of adiponectin by adipose tissue increases during starvation and is inversely related in obese individuals to visceral adiposity and BMI [
1,
37,
38]. Furthermore, adiponectin increases insulin sensitivity. Decreases in adiponectin levels result in insulin resistance and metabolic syndrome. Adiponectin levels have been found to be low in obese females [
1].
Adiponectin has been found in circulation in various molecular isoforms. Four-fifths of circulating adiponectin is the high-molecular-weight isoform. The level of adiponectin is nearly double in serum when compared with that in human seminal plasma. This is hypothesized to be due to the blood–testis barrier. The measured adiponectin level in semen is lower in overweight and obese patients when compared with patients with normal BMIs [
5,
42,
43].
The level of circulating adiponectin is inversely proportional to the level of circulating testosterone [
44]. Possibly because of the low serum concentrations of testosterone seen in obese males, levels of serum adiponectin have been shown to be paradoxically elevated in obese males. This suggests a resistance phenomenon similar to insulin and leptin in obese males [
11].
3.3. Resistin
Resistin is a polypeptide that is involved in the development of insulin resistance. The modulation of insulin sensitivity and the differentiation of adipocytes are among the known functions of resistin [
37]. Increased concentrations of resistin have been correlated with low sperm motility and vitality. However, other studies have not shown any effect of resistin on sperm function [
43].
Resistin is also associated with increased levels of cytokines such as interleukin-6 and elastase in seminal fluid and is implicated in the inflammation of the reproductive organs noted in obese males [
5,
45]. Apart from the peripheral effects of resistin on the reproductive system in obese males, resistin has also been isolated from the pituitary gland and hypothalamus in humans, suggesting a central action too [
5,
46].
3.4. Ghrelin
Ghrelin is an orexigenic peptide released from the stomach during fasting states and has been called the “hunger hormone” [
11,
13]. Circulating levels of ghrelin have been shown to be inversely correlated with BMI. When administered centrally, ghrelin has been shown to increase the utilization of glucose in adipose tissue. It has been shown to protect the testes and sperm from endoplasmic reticulum stress and inhibit the inflammatory response to stress and apoptosis [
47].
Ghrelin in association with leptin is involved in the control of adiposity in obese individuals. Ghrelin has been found in Leydig and Sertoli cells in the testes and inhibits testosterone secretion. It also plays a role in the regulation of spermatogenesis, and decreased levels may suppress the secretion of FSH and LH in adult men [
13,
48,
49,
50,
51]. Low serum concentrations of ghrelin have been found to adversely decrease semen volume, sperm motility, and morphology [
52].
3.5. Chemerin
Chemerin is another peptide secreted by adipocytes that modulates insulin [
5]. Concentrations of chemerin are elevated in obese individuals when compared with normal-weight individuals, and its levels are directly correlated with increases in BMI and inversely correlated with levels of testosterone [
43]. Chemerin and its receptors have been found in the testes and adipose tissue and have adverse effects on testicular steroidogenesis. Chemerin effects the release of gonadotropins from the hypothalamus and is concentrated in the Leydig cells. Anti-androgens have been demonstrated to inhibit the expression of chemerin in Leydig cells [
53,
54,
55]. Adiponectin and chemerin are called “contrary adipokines”, as they display opposing functions, where adiponectin promotes insulin sensitivity while chemerin inhibits the signaling of insulin. A high-adiponectin-to-low-chemerin ratio is suggested to play an important role in the development of metabolic syndrome in obesity, and this might be a target of therapeutic interventions meant to treat infertility in obese males [
56].
3.6. Visfatin
Visfatin, which is also known as a pre-B-cell colony-enhancing factor or phosphoribosyltransferase, is a protein whose role in obesity is uncertain [
5]. It has been theorized to be an insulin-like adipocytokine secreted from adipose cells. Protective and adverse roles in obese individuals have both been reported. More recently, it has been reported to produce proinflammatory expression in genes, including the
CD68 and
tumor necrosis factor-alpha (TNF-α) genes in adipose tissue, and is associated with insulin resistance and hyperlipidemia [
57,
58,
59]. Visfatin is found in seminal fluid, Leydig cells, and spermatozoa and has been shown to increase testosterone levels [
60,
61].
3.7. Apelin, Omentin, Hepcidin, and Vaspin
Apelin increases with adiposity and with increased insulin levels. It modulates insulin resistance by increasing the expression of uncoupling proteins in adipose tissue and decreasing the levels of adiponectin [
62,
63]. Omentin-1 seems to have an anti-inflammatory and antioxidative action [
64]. Levels of omentin-1/intelectin, like adiponectin, decrease with insulin resistance, BMI, and adiposity [
62,
65]. Hepcidin seems to modulate inflammatory cytokines like TNF-α and C-reactive protein, and its concentrations have been found to increase in obese individuals [
66]. Vaspin is a serine protease inhibitor that is also an insulin sensitizer and has increased concentrations in adipose individuals, especially those with increased glucose tolerance [
62].
4. Reproductive Steroids
4.1. Hypothalamic–Pituitary–Testicular Axis
Normal reproductive hormone secretion is imperative for normal male fertility. Infertility may result from any interference with the intricately balanced interplay between the constituents of the hypothalamic–pituitary–testicular axis [
67,
68].
The hypothalamic–pituitary–testicular axis regulates the production of testosterone, which, in turn, is influenced by direct negative feedback from testosterone. LH and FSH are secreted in response to the pulsatile release of gonadotropin-releasing hormone (GnRH). LH stimulates testosterone secretion by acting on Leydig cells, and FSH induces spermatogenesis by acting on Sertoli cells in the testes [
5,
7].
The effects of obesity and hypogonadism form a vicious cycle, whereby dysregulation of the hypothalamic–pituitary–testicular axis—due to the effect of the release of multiple mediators, thus decreasing GnRH release from the GnRH neurons of the hypothalamus—causes decreases in LH and FSH levels. This leads to lower levels of testosterone, which further increases adiposity because of increased lipogenesis [
69].
Since the 1970s, researchers have identified hormonal differences between obese and non-obese adult males in the hypothalamic–pituitary–testicular axis. Adult obese males have been found to have decreased peripheral levels of total testosterone and sex hormone-binding globulin (SHBG). Lower levels of SHBG are mostly mediated by elevated levels of circulating insulin linked to the insulin resistance of obesity [
25]. The significantly decreased SHBG concentration is the primary cause of the concurrent decrease in total testosterone [
70]. There is a negative correlation between the degree of obesity, as measured by the BMI, and levels of total testosterone and SHBG [
7,
17,
67,
71,
72]. The typical reproductive hormonal profile in obese adult males is that of hyperestrogenic hypogonadotropic hypoandrogenemia [
25].
4.2. Estrogens
The peripheral aromatization of androgens leads to elevated levels of estrogens. Increased adiposity in obese males boosts conversion rates of testosterone into estradiol [
11,
25,
37]. Estradiol blunts GnRH pulses in the hypothalamus and, therefore, FSH and LH secretion in the pituitary. The rise in estradiol levels in obese men reduces the production of FSH and LH, which impairs testicular function and lowers levels of testosterone [
25].
Kisspeptin neurons in the hypothalamus act as a center for the regulation of reproductive hormones. Numerous regulatory factors govern the release of kisspeptin from these neurons, which, in turn, affects the release of GnRH from GnRH neurons that control the reproductive axis. Estrogens produce negative feedback in the arcuate nucleus of the hypothalamus, which specifically decreases the production of kisspeptin, thereby inhibiting the pulsatile release of GnRH, resulting in decreased production of FSH and LH from the pituitary gland [
11,
73,
74].
4.3. Inhibin B
Inhibin B is a protein dimer composed of an alpha and a beta subunit produced primarily by Sertoli cells in the prepubertal testes. The site of secretion in adult males is still not clear. Inhibin B regulates FSH production via a negative feedback mechanism and, in turn, is regulated by FSH and LH levels. There is a positive correlation between testicular volume and sperm production with the level of circulating inhibin B. This makes the measurement of inhibin B a good marker of male reproductive function [
75,
76,
77]. Inhibin B is considered to be a marker for “nurse” Sertoli cells, which are primarily involved in the nutrition and physical support of spermatogenesis [
13].
5. Incretins
Glucagon-like peptide-1 (GLP-1), which is an incretin hormone, is responsible for the control of insulin release from the pancreatic islet in response to meal ingestion and consequent glucose loading. It has a very short half-life of 1.5 to 5 min and is rapidly cleared by the kidney [
78]. It crosses the blood–brain barrier and induces satiety, thereby reducing food intake. Low levels of GLP-1 have been shown to reduce the testosterone pulse frequency and reduce testicular weight [
13]. The administration of GLP-1 receptor antagonists has been demonstrated to increase testosterone levels and increase testicular weight [
79].