Synthetic plastics are commonly used because they exhibit sufficient characteristics for packaging requirements, but their end lives result in environmental pollution, the depletion of landfill space, rising sea pollution, and more. These exist because of their poor biodegradability, limited recyclability, etc. There has been an increasing demand for replacing these polymers with bio-based biodegradable materials for a sustainable environment. Cellulosic nanomaterials have been proposed as a potential substitute in the preparation of packaging films. Cellulose can be extracted from different sources, such as wood, agricultural by-products, annual plants, and marine algae. There are two forms of nanocellulose extracted from plant fibers: cellulose nanofibers (CNFs) and cellulose nanocrystals (CNCs).
- cellulose
- nanocellulose
1. Introduction
2. Preparation of Nanocellulose
2.1. Pretreatment Methods of Lignocellulosic Biomass
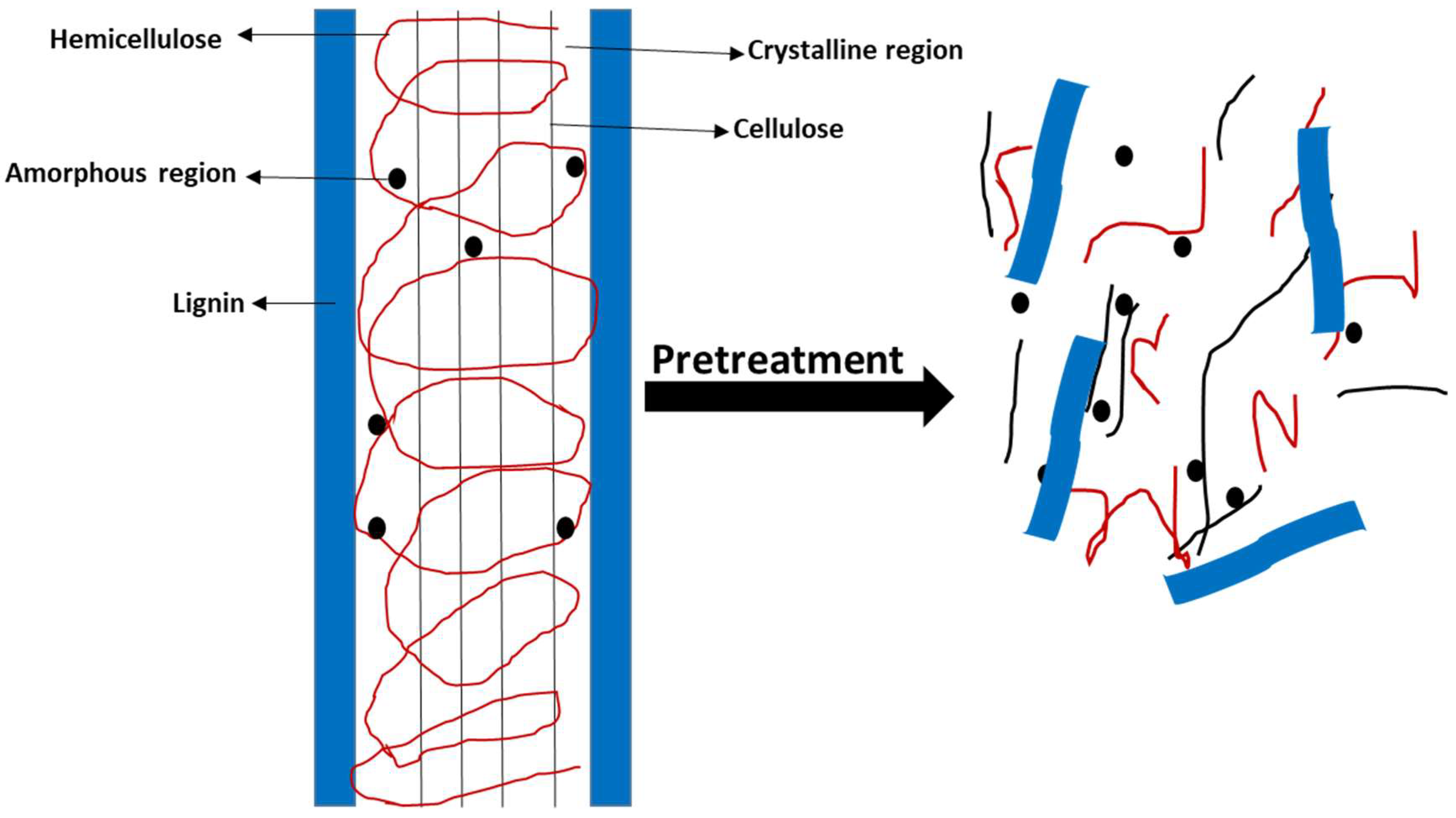
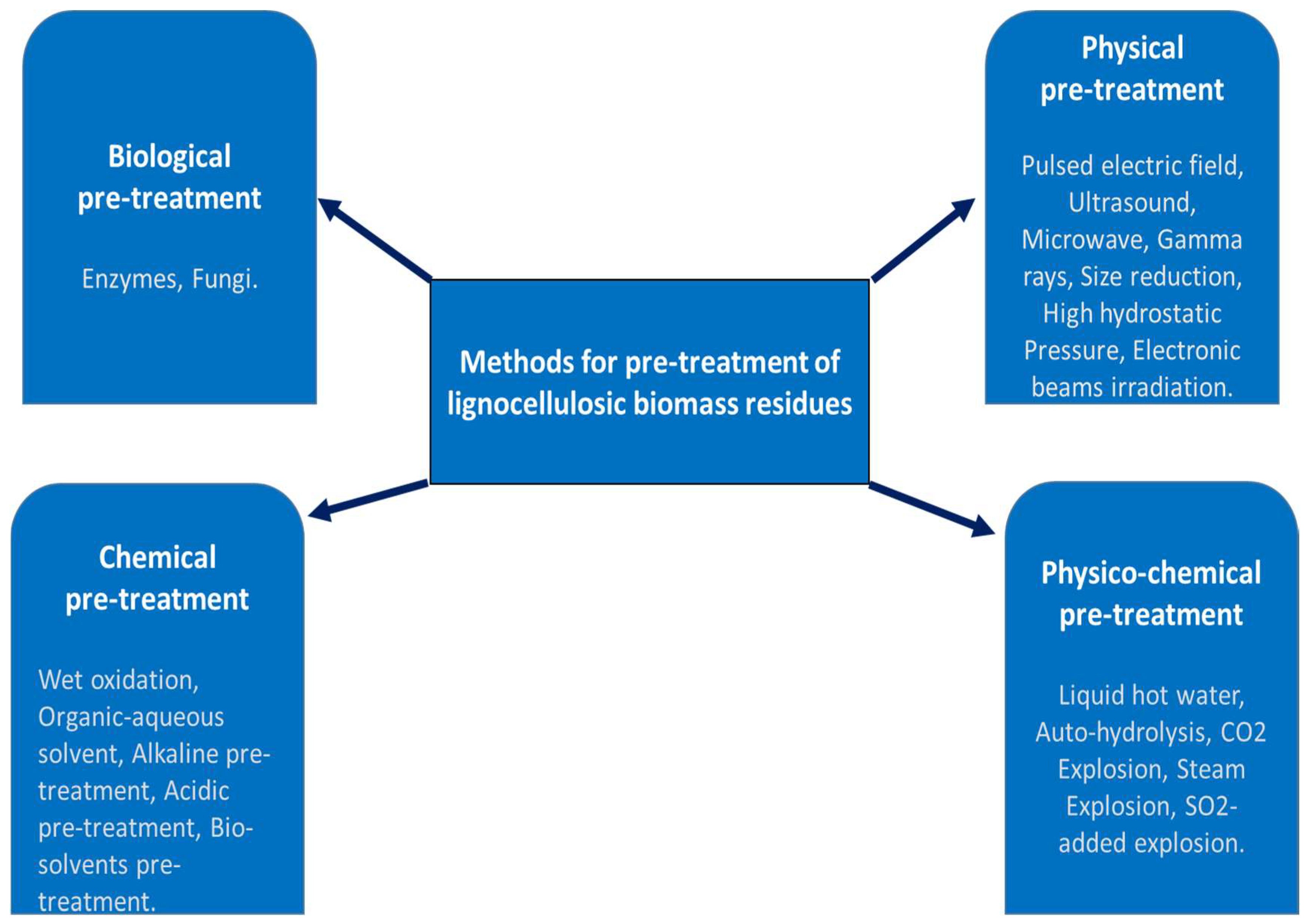
2.1.1. Biological Methods
2.1.2. Physical Methods

2.1.3. Physicochemical Methods
2.1.4. Chemical Methods
2.2. Bleaching

2.3. Methods for Cellulose Nanoparticle Isolation
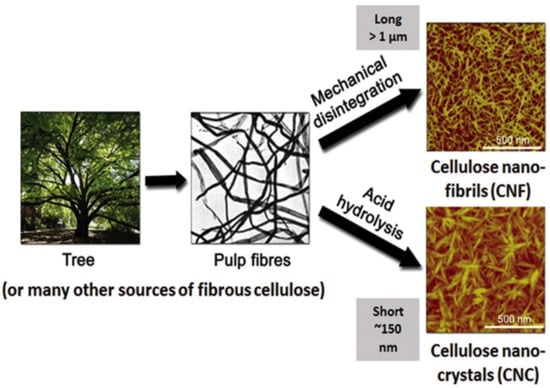
2.3.1. Physical Methods
2.3.2. Enzymatic Hydrolysis
2.3.3. Acid Hydrolysis
This entry is adapted from the peer-reviewed paper 10.3390/polym16010051
References
- Lindström, T.; Österberg, F. Evolution of biobased and nanotechnology packaging—A review. Nord. Pulp Pap. Res. J. 2020, 35, 491–515.
- Bardet, R.; Reverdy, C.; Belgacem, N.; Leirset, I.; Syverud, K.; Bardet, M.; Bras, J. Substitution of nanoclay in high gas barrier films of cellulose nanofibrils with cellulose nanocrystals and thermal treatment. Cellulose 2015, 22, 1227–1241.
- Abelti, A.L.; Teka, T.A.; Fikreyesus Forsido, S.; Tamiru, M.; Bultosa, G.; Alkhtib, A.; Burton, E. Bio-based smart materials for fish product packaging: A review. Int. J. Food Prop. 2022, 25, 857–871.
- Palali, S.N. Cellulose Nanocrystals: Potential Replacement for Food Packaging. Science 2019, 39–73.
- Romão, S.; Bettencourt, A.; Ribeiro, I.A. Novel Features of Cellulose-Based Films as Sustainable Alternatives for Food Packaging. Polymers 2022, 14, 4968.
- Ohlrogge, J.; Allen, D.; Berguson, B.; DellaPenna, D.; Shachar-Hill, Y.; Stymne, S. Driving on biomass. Science 2009, 324, 1019–1020.
- Abdullah, J.A.A.; Jiménez-Rosado, M.; Guerrero, A.; Romero, A. Biopolymer-Based Films Reinforced with Green Synthesized Zinc Oxide Nanoparticles. Polymers 2022, 14, 5202.
- Cakmak, H.; Dekker, M. Optimization of Cellulosic Fiber Extraction from Parsley Stalks and Utilization as Filler in Composite Biobased Films. Foods 2022, 11, 3932.
- Fang, Z.; Hou, G.; Chen, C.; Hu, L. Nanocellulose-based films and their emerging applications. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100764.
- Li, J.; Song, Z.; Li, D.; Shang, S.; Guo, Y. Cotton cellulose nanofiber-reinforced high density polyethylene composites prepared with two different pretreatment methods. Ind. Crops Prod. 2014, 59, 318–328.
- Rodríguez-Fabià, S.; Torstensen, J.; Johansson, L.; Syverud, K. Hydrophobization of lignocellulosic materials part III: Modification with polymers. Cellulose 2022, 29, 5943–5977.
- Boluk, Y.; Lahiji, R.; Zhao, L.; McDermott, M.T. Suspension viscosities and shape parameter of cellulose nanocrystals (CNC). Colloids Surf. A Physicochem. Eng. Asp. 2011, 377, 297–303.
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494.
- Hua, K.; Carlsson, D.O.; Ålander, E.; Lindström, T.; Strømme, M.; Mihranyan, A.; Ferraz, N. Translational study between structure and biological response of nanocellulose from wood and green algae. RSC Adv. 2014, 4, 2892–2903.
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969.
- Poulose, A.; Parameswaranpillai, J.; George, J.J.; Gopi, J.A.; Krishnasamy, S.; Dominic, C.D.M.; Hameed, N.; Salim, N.V.; Radoor, S.; Sienkiewicz, N. Nanocellulose: A Fundamental Material for Science and Technology Applications. Molecules 2022, 27, 8032.
- Wang, C.; Shi, J.; He, M.; Ding, L.; Li, S.; Wang, Z.; Wei, J. High strength cellulose/ATT composite films with good oxygen barrier property for sustainable packaging applications. Cellulose 2018, 25, 4145–4154.
- Ai, B.; Zheng, L.; Li, W.; Zheng, X.; Yang, Y.; Xiao, D.; Shi, J.; Sheng, Z. Biodegradable cellulose film prepared from banana pseudo-stem using an ionic liquid for mango preservation. Front. Plant Sci. 2021, 12, 625878.
- Taherimehr, M.; YousefniaPasha, H.; Tabatabaeekoloor, R.; Pesaranhajiabbas, E. Trends and challenges of biopolymer-based nanocomposites in food packaging. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5321–5344.
- Nair, S.S.; Zhu, J.; Deng, Y.; Ragauskas, A.J. High performance green barriers based on nanocellulose. Sustain. Chem. Process. 2014, 2, 1–7.
- Sethi, J.; Wågberg, L.; Larsson, P.A. Water-resistant hybrid cellulose nanofibril films prepared by charge reversal on gibbsite nanoclays. Carbohydr. Polym. 2022, 295, 119867.
- Yu, Z.; Ji, Y.; Bourg, V.; Bilgen, M.; Meredith, J.C. Chitin-and cellulose-based sustainable barrier materials: A review. Emergent Mater. 2020, 3, 919–936.
- Abdul Khalil, H.P.S.; Chong, E.W.N.; Owolabi, F.A.T.; Asniza, M.; Tye, Y.Y.; Rizal, S.; Nurul Fazita, M.R.; Mohamad Haafiz, M.K.; Nurmiati, Z.; Paridah, M.T. Enhancement of basic properties of polysaccharide-based composites with organic and inorganic fillers: A review. J. Appl. Polym. Sci. 2019, 136, 47251.
- Saedi, S.; Garcia, C.V.; Kim, J.T.; Shin, G.H. Physical and chemical modifications of cellulose fibers for food packaging applications. Cellulose 2021, 28, 8877–8897.
- Hussin, F.N.N.M.; Wahab, R.A.; Attan, N. Nanocellulose and nanoclay as reinforcement materials in polymer composites: A review. Malays. J. Fundam. Appl. Sci. 2020, 16, 145–153.
- Mdletshe, G.P. Extraction and Characterisation of Cellulose Nanocrystals (CNCs) from Sugarcane Bagasse Using Ionic Liquids. Ph.D. Thesis, Durban University of Technoloy, Durban, South Africa, 2019.
- Beig, B.; Riaz, M.; Naqvi, S.R.; Hassan, M.; Zheng, Z.; Karimi, K.; Pugazhendhi, A.; Atabani, A.; Chi, N.T.L. Current challenges and innovative developments in pretreatment of lignocellulosic residues for biofuel production: A review. Fuel 2021, 287, 119670.
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A.; Karim, S.; Yu, G. Insight into progress in pre-treatment of lignocellulosic biomass. Energy 2017, 122, 724–745.
- Das, N.; Jena, P.K.; Padhi, D.; Kumar Mohanty, M.; Sahoo, G. A comprehensive review of characterization, pretreatment and its applications on different lignocellulosic biomass for bioethanol production. Biomass Convers. Biorefin. 2021, 13, 1503–1527.
- Saritha, M.; Arora, A.; Lata. Biological Pretreatment of Lignocellulosic Substrates for Enhanced Delignification and Enzymatic Digestibility. Indian J. Microbiol. 2012, 52, 122–130.
- Cheng, J.J.; Timilsina, G.R. Status and barriers of advanced biofuel technologies: A review. Renew. Energy 2011, 36, 3541–3549.
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess 2017, 4, 7.
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11.
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65.
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729.
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140.
- Sarkar, N.; Ghosh, S.K.; Bannerjee, S.; Aikat, K. Bioethanol production from agricultural wastes: An overview. Renew. Energy 2012, 37, 19–27.
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and physicochemical pretreatment of lignocellulosic biomass: A review. Enzym. Res 2011, 2011, 787532.
- Teixeira, R.S.S.; Silva, A.S.; Moutta, R.O.; Ferreira-Leitão, V.S.; Barros, R.R.O.; Ferrara, M.A.; Bon, E.P.S. Biomass pretreatment: A critical choice for biomass utilization via biotechnological routes. BMC Proc. 2014, 8, O34.
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206.
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106.
- Gáspár, M.; Kálmán, G.; Réczey, K. Corn fiber as a raw material for hemicellulose and ethanol production. Process Biochem. 2007, 42, 1135–1139.
- Zhao, Y.; Wang, J.; Liu, X.; Zhang, S. Effects of cationic structure on cellulose dissolution in ionic liquids: A molecular dynamics study. ChemPhysChem 2012, 13, 3126–3133.
- Feng, L.; Chen, Z.-L. Research progress on dissolution and functional modification of cellulose in ionic liquids. J. Mol. Liq. 2008, 142, 1–5.
- Aita, G.M.; Kim, M. Pretreatment Technologies for the Conversion of Lignocellulosic Materials to Bioethanol. In Sustainability of the Sugar and Sugar−Ethanol Industries; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2010; pp. 117–145.
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.M. Hemicellulose biorefineries: A review on biomass pretreatments. J. Sci. Ind. Res. 2008, 67, 849–864.
- Singh, L.K.; Chaudhary, G.; Majumder, C.; Ghosh, S. Utilization of hemicellulosic fraction of lignocellulosic biomaterial for bioethanol production. Adv. Appl. Sci. Res. 2011, 2, 508–521.
- Ng, H.-M.; Sin, L.T.; Tee, T.-T.; Bee, S.-T.; Hui, D.; Low, C.-Y.; Rahmat, A.R. Extraction of cellulose nanocrystals from plant sources for application as reinforcing agent in polymers. Compos. Part B Eng. 2015, 75, 176–200.
- Hazwan Hussin, M.; Trache, D.; Chuin, C.T.H.; Nurul Fazita, M.; Mohamad Haafiz, M.; Hossain, M. Extraction of cellulose nanofibers and their eco-friendly polymer composites. In Sustainable Polymer Composites and Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2019; pp. 653–691.
- Pereira, A.L.S.; Nascimento, D.M.; Cordeiro, E.M.S.; Morais, J.P.S.; Sousa, M.d.S.M.; Rosa, M.d.F. Characterization of Lignocellulosic Materials Extracted from the Banana Pseudostem. 2010. Available online: https://ainfo.cnptia.embrapa.br/digital/bitstream/item/34240/1/AT10096.pdf (accessed on 5 April 2023).
- Aridi, A.S.; Chin, N.L.; Ishak, N.A.; Yusof, N.N.M.; Kadota, K.; Manaf, Y.N.; Yusof, Y.A. Effect of Sodium Hypochlorite Concentration during Pre-treatment on Isolation of Nanocrystalline Cellulose from Leucaena leucocephala (Lam.) Mature Pods. BioResources 2021, 16, 3137.
- Shin, H.K.; Pyo Jeun, J.; Bin Kim, H.; Hyun Kang, P. Isolation of cellulose fibers from kenaf using electron beam. Radiat. Phys. Chem. 2012, 81, 936–940.
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392.
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54.
- Marakana, P.G.; Dey, A.; Saini, B. Isolation of nanocellulose from lignocellulosic biomass: Synthesis, characterization, modification, and potential applications. J. Environ. Chem. Eng. 2021, 9, 106606.
- Tahir, D.; Karim, M.R.A.; Hu, H.; Naseem, S.; Rehan, M.; Ahmad, M.; Zhang, M. Sources, Chemical Functionalization, and Commercial Applications of Nanocellulose and Nanocellulose-Based Composites: A Review. Polymers 2022, 14, 4468.
- Trache, D.; Hussin, M.H.; Haafiz, M.M.; Thakur, V.K. Recent progress in cellulose nanocrystals: Sources and production. Nanoscale 2017, 9, 1763–1786.
- Rana, A.K.; Frollini, E.; Thakur, V.K. Cellulose nanocrystals: Pretreatments, preparation strategies, and surface functionalization. Int. J. Biol. Macromol. 2021, 182, 1554–1581.
- Piras, C.C.; Fernández-Prieto, S.; De Borggraeve, W.M. Ball milling: A green technology for the preparation and functionalisation of nanocellulose derivatives. Nanoscale Adv. 2019, 1, 937–947.
- Ferreira, R.R.; Souza, A.G.; Nunes, L.L.; Shahi, N.; Rangari, V.K.; dos Santos Rosa, D. Use of ball mill to prepare nanocellulose from eucalyptus biomass: Challenges and process optimization by combined method. Mater. Today Commun. 2020, 22, 100755.
- Shokrkar, H.; Ebrahimi, S.; Zamani, M. A review of bioreactor technology used for enzymatic hydrolysis of cellulosic materials. Cellulose 2018, 25, 6279–6304.
- Yang, B.; Dai, Z.; Ding, S.-Y.; Wyman, C.E. Enzymatic hydrolysis of cellulosic biomass. Biofuels 2014, 2, 421–449.
