Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Microplastics (MPs) are small plastic particles that are less than 5 mm in size, and they have become a significant environmental concern due to their ubiquitous presence in the environment.
- microplastics
- instrumental analysis
- agriculture
- FTIR
- SEM
- raman
1. Introduction
Microplastics (MPs) are small plastic particles that are less than 5 mm in size, and they have become a significant environmental concern due to their ubiquitous presence in the environment. These plastics have been detected in marine and terrestrial environments [1,2]. MPs persist in the environment for decades, are often ingested by wildlife, and may have negative impacts on ecosystems and human health by being a vector carrying toxic chemicals.
One of the major reasons why microplastic (MP) pollution in the agricultural environment has been overlooked is the unavailability of appropriate and unified analytical techniques [3]. To understand the extent and effects of microplastic pollution, it is important to accurately quantify and identify MPs in different environmental matrices because plastics have varying properties and composition depending on the polymer type. The detection and quantification of MPs in environmental samples have gone past physical analysis using methods such as floatation and hand picking. MPs analysis requires advanced analytical techniques due to their small size and low concentration. Chemical analysis has been able to evolve over the years from wet analysis methods (destructive analysis) to the use of modern analytical instruments (non-destructive analysis) [4].
Fourier transform infrared (FTIR) spectroscopy and Raman spectroscopy are non-destructive powerful techniques for the identification of MPs based on their chemical composition and structure in different environments; however, these instruments have limitations such as cost and high-expertise analysts to efficiently run the analysis and interpret data [3,5]. Fourier transform infrared (FTIR) spectroscopy is a widely used technique for the analysis of MPs due to its high sensitivity and specificity. This technique involves the measurement of the absorbance or transmission of infrared radiation by a sample. For example, the presence of a carbonyl group in the microplastic structure can be detected by the peak at 1700 cm−1 [6]. Raman spectroscopy technique involves the measurement of the scattered light from a sample when exposed to a laser beam. Raman spectroscopy can provide information on the chemical composition and structure of MPs based on their unique Raman spectra. This technique has been used to analyze MPs in seawater, sediment, and biota samples.
Pyrolysis–Gas Chromatography–Mass Spectroscopy (Pyr/GC/MS) is an analytical technique that involves the thermal degradation of a sample under controlled conditions followed by the separation and identification of the pyrolysis products by gas chromatography and mass spectrometry [5]. It is limited to destroying the sample in the pyrolysis process though chromatography techniques are highly sensitive and can provide quantitative information on the chemical composition of MPs. Flow cytometry (FC) can provide information on the size distribution and concentration of MPs in a sample. FC has been evidently used in analyzing MPs in aquatic environments but not in soil and compost matrices. Microscopy techniques have been widely used for the detection and characterization of MPs [7]. Optical microscopy, such as stereomicroscopy and polarized light microscopy (PLM), is often used to identify and count particles based on their visual characteristics. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) can provide higher-resolution images for the analysis of the surface structure and morphology of MPs [8]. Previous research has shown the advantages and disadvantages of these techniques in experimental applications in terms of cost–benefit effectiveness, the purpose of the project, turnaround time, expected outcomes, and the accuracy/recovery rate.
2. Instrumental Analysis in Soil and Compost Microplastic Assessment
Researchers used a systematic literature search to sort out scientific studies containing primary data on microplastic instrumental analysis in two agricultural matrices (soil and compost). Researchers focused on those studies reflecting non-destructive (FTIR, SEM) and destructive (pyr/GC/MS, TED/GC/MS) methods of analysis (Figure 1), to highlight their merits and demerits as fitting with the cost, time, and purpose of the project. When choosing the analytical techniques, researchers must intentionally consider some factors such as the aim/purpose of the project, study size, expected outcomes, turnaround time, and especially the type of analysis. These factors will inform the most suitable instrument to utilize in the analysis [6]. Similarly, the chemistry in terms of sample preparation prior to analysis influences the nature of sample characterization [9]. Hence, it could result in a destructive technique where the sample is either crushed, digested/dissolved, or even mixed with other substances prior to characterization. In some cases, it could be non-destructive because the sample is still kept intact while undergoing characterization. Either condition is peculiar to individual instruments used in the analysis of simple compounds or complex polymers such as MPs.
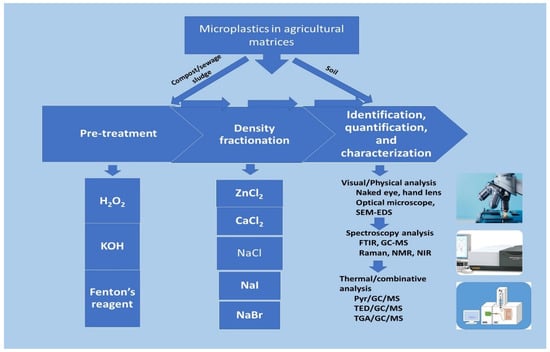
Figure 1. Microplastics analytical techniques and methods.
Figure 1 shows the two major methods of MPs analysis in agricultural matrices where MPs in the soil can be easily identified without pretreatment, but compost and sewage samples are treated in a combinative approach of organic digestive, density fractionation, sieving before instrumental analysis is carried out for characterization. H2O2—hydrogen peroxide; KOH—potassium hydroxide; SEM-EDS—scanning electron microscopy–energy dispersive X-ray spectroscopy; GC-MS—gas chromatography–mass spectroscopy; FTIR—Fourier transform infrared; NMR—nuclear magnetic resonance; NIR—near infrared; Pyr/GC/MS—pyrolysis/gas chromatography–mass spectroscopy; TED/GC/MS—thermal extraction and desorption/gas chromatography–mass spectroscopy, TGA/GC/MS—Thermogravimetric gas/gas chromatography–mass spectroscopy.
2.1. Microplastics in Agricultural Matrices
Agricultural matrices could be complex as they could involve various components of an ecosystem. However, the core of agricultural systems, especially farming, is soil and soil amendments such as compost. Microplastic in the soil as an environmental pollution has been gaining more attention recently. Since studies have revealed the poor degradation characteristics of plastics [10], larger sizes of plastics remaining in the soil break down into smaller particles [11,12]. Some research has confirmed the presence of MPs in soils [2,3,13,14,15,16]. There are diverse ways through which MPs enter the soil and sewage sludge and compost application are involved [17,18,19]. Terrestrial environments, especially agricultural soils, are more vulnerable to heavy MP pollution due to the application of soil enhancements as well as direct contact with other anthropogenic activities. Despite significant research carried out on soil MPs, knowledge paucity remains an issue [7], though there is more published research on soil MPs than with microplastic research on compost, which is a potential source and pathway for plastic pollution into the soil. For the assessment of MP in soil and compost, there has been a lack of universal procedures and protocols in the sample collection and analysis (Table 1 and Table 2). This lack of standardization is a huge cause for limited knowledge in this field of study [20].
Table 1. Analytical methods of microplastics in soil.
| Separation Method | Extracting Solution | Extraction | Repeat | Clean up | Instrumental Analysis | Quantification | Ref. |
|---|---|---|---|---|---|---|---|
| Stir for 30 min, ultrasound for 2 min, settling for 24 h | NaCl (1.19 g/L) | DF | 3 times | H2O2 (30%) | Microscopy—VI, µ-FTIR | Counting | [14] |
| Floatation, filtration, ultrasound for 2 h, heating | DW | Floatation | >4 times | Filtration | Microscopy—VI | Weighing | [21] |
| Stir for 30 min, settling for 24 h | NaCl (1.19 g/L) | DF | 3 times | H2O2 (30%) | Microscopy—VI, µ-FTIR | Counting | [15] |
| Ultrasound treatment for 20 min | NaI (1.8 g/L) | DF | >2 times | H2O2 (35%), NaOH (0.5 M) | Microscopy—VI | Counting | [2] |
| Stir and centrifuge | DW, NaCl (1.20 g/L), ZnCl2 (1.55 g/L) | DF | 3 times | Stereomicroscope—VI | Counting | [22] | |
| Sedimentation cylinder method, use of MP separator, stir for 10 min, then centrifuge for 30 min | NaCl (1.2 g/L), CaCl2 (1.5 g/L) |
DF | 3 or 4 times | KCIO (13%) NaOH (50%) H2SO4 (96%) HNO3 (65%) H2O2 (30%) |
Raman Spectrometry, FTIR | Weighing | [3] |
| Stir, centrifuge, and floatation | NaCl, NaOH | Floatation | NIR spectroscopy | Weighing | [23] |
VI—Visual Inspection; μ-FTIR—micro-FTIR; NaCl—Sodium chloride; CaCl2—Calcium chloride; NaOH—Sodium hydroxide; H2O2—Hydrogen peroxide; ZnCl2—Zinc chloride; NaI—Sodium iodide; KCIO—Potassium hypochlorite; HF—hydrogen fluoride; C2H6O—Ethanol; HNO3—nitric acid; H2SO4—Sulfuric acid; DF—Density fractionation; DW—Distilled water.
Table 2. Analytical methods of microplastics in mixed soil and other matrices.
| Matrix | Separation Method | Extracting Solution | Extraction | Repeat | Clean Up | Instrumental Analysis | Quantification | Ref. |
|---|---|---|---|---|---|---|---|---|
| Spiked soil | Overnight drying | HNO3 (10%), C2H6O | TGA-FTIR spectroscopy | [24] | ||||
| Soil + MSW Compost | Shaking and sieving, sedimentation and siphoning, centrifugation | Water | WF | 8 | HF | TED-EDX—VI, Pyr/GC/MS | Weighing | [25] |
| Soil + Compost | ZnCl2 | DS | Microscopy—VI | Counting | [26] | |||
| Soil + Manure | Ultrasonic for 10 min, stir for 30 min, settling for 24 h, centrifuged for 30 min | H2O2 (30%) | DS | 3 | SEM—VI | [20] | ||
| Treated Compost | KCOOH | DS | Fenton’s reagent | VI, FTIR spectrometry, Fluorescence microscopy, Nile Red Dye Staining | [27] | |||
| Soil, compost | Centrifuged, sieving | Methanol, water, liquid nitrogen | WF | H2O2 | TED/GC/MS, NIR spectrometry | [9] | ||
| Soil | Sieving | ZnCl2 (1.58 g/L) | DS | FTIR, Hyperspectral imaging | [28] | |||
| Spiked soil, soil | Filtration, sieving | NaBr (1.55 g/L) | DS/LS, Filtration | Fenton’s reagent | Microscopy visual identification, Nile Red staining, ICP-MS, ATR-FTIR | Weighing | [29] | |
| Farmland soil | Stir for 15 min, settle for 30 min | NaCl (1.2 g/L) | DS | KOH (10%) | SEM, ATR-FTIR spectroscopy, Pyr-GC-MS, ICP-MS | [30] | ||
| Soil around waste facility | Stir for 10 min, settling for 24 h | NaCl (1.2 g·cm−3) | DS | 3 times | H2O2 (30%) | Microscope—VI, Raman micro-spectroscopy, SEM-EDS | Weighing | [31] |
| Soil | Stir for 30 min, settle for 12 h until suspension is clear | NaCl | DS | 3 or 4 times | Deionized water | Hyperspectral imaging | [32] |
VI—Visual Inspection; KCOOH—Potassium formate; NaCl—Sodium Chloride; NaBr—Sodium bromide; H2O2—Hydrogen peroxide; ZnCl2—Zinc chloride; DS—Density separation; WF—Water fractionation; LS—Lipophilic separation.
To assess MPs in any environmental matrix, samples from the matrices are collected for analysis. There have been different steps in assessing MPs in soil: extraction, washing/cleaning, identification, and quantification. However, there are two major methods of assessing MPs: the physical counting/observation and the instrumental analysis (Figure 1). Some of the recent instrumental methods can directly detect/identify MPs without extraction and/or cleaning [33]. To achieve quantification/counting of MPs in compost samples, the presence of organic matter must be sorted [34,35]. Usually, this is taken care of during the process of cleaning by using the protocol where after water separation and sonication are performed on the compost samples, 30 mL of hydrogen peroxide (30% w/v) is added to 5 g of the compost and covered in an oven at 60 °C overnight [33,36], sulfuric acid (H2SO4) [37], KCIO, HNO3, NaOH [3], and Ethanol [24]. Fenton reagent (H2O2 + Fe (II) catalyst) can also be used to digest organic materials effectively [38,39,40,41]. This process helps remove the organic matter content and non-plastic organic materials that could interfere with visual and/or spectroscopic analysis. The major difference between analyzing soil and compost MPs is the combination of elutriation, sieving, oxidative treatment, and density separation carried out in compost in comparison to elutriation and/or density separation in the soil matrix. There is little research on MP characterization from organic sources, and one of the causes of this is that many instrumental techniques have their efficiency reduced by interference of impurities [27]. In addition, extensive pretreatment or preparation of samples adds to the long analysis run time, making the procedure take even longer time. Also, there is an issue with obtaining a 3-dimensional heterogeneity with plastic pollution in soil and the inability to obtain robust data on soil, compost or sewage sludge samples, as they are multifarious and complex matrices [41,42,43]. Density separation, which is a major method of sample extraction in samples containing heavy OM and other impure particles (Table 1), is yet to be confirmed as an appropriate method of MP extraction [44]. In addition, this method lacks standardization in both the choice of salt solution and the densities. Despite the emergence of these advanced tools, including hyperspectral imaging, extraction, and analysis of MPs in soil and other agricultural matrices may remain challenging. This is possible because of the varying disadvantages associated with organic matter present in these samples, and the longer preparation time compared to analyzing MPs from aquatic environments.
One of the major problems facing microplastic assessment in soil matrix is the lack of a standardized analytical approach which inhibits the progress of identifying a unified solution to microplastic pollution [45,46]. However, the classification of analytical techniques into destructive and non-destructive as well as classification according to technique has been established [Figure 1 and Figure 2]. For instance, Figure 1 illustrates that microscopic analysis (SEM) and FTIR spectroscopic techniques are non-destructive, while Figure 2 categorized MP instrumental analysis into advanced technologies, microscopic, spectroscopic, thermal. Non-destructive techniques are the analytical methods that are used to analyze samples without destroying their surface texture, structure, shape, color, general integrity, and usefulness; the originality remains intact. In contrast, destructive techniques interfere with the originality of the samples by converting them to a more suitable form for the analysis. Examples are thermo-analytic technologies such as the pyr/GC/MS, and thermo-extraction desorption–gas chromatography–mass spectrometry TED/GC/MS).
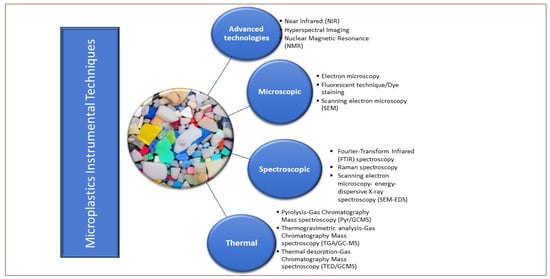
Figure 2. Classification of instrumental techniques in microplastic study.
2.2. Visual Inspection
Visual analysis is a basic method of physical characterization in MP assessment [47]. Following the steps involved in MPs analysis in soil [21,26,29,31,48,49] and compost [20,25,27,36], the identification of MPs visually offers quite a simple approach to both scientists and non-experts [50]. The naked eye identification, magnifying hand lens, and stereomicroscopes with an attachment of professional imaging software [2,14], as well as magnifying hand lens, are commonly used in optical identification and separation of MPs (1 mm–5 mm) from soil and composts. Heat can also be applied to microscopy [51]; however, the experiment has limitations, as this effective thermal application was only verifiable on polyethylene (PE) and polypropylene (PP) from soil. There are several merits of the visual method of assessment, and it includes ease of carrying it out with little or no professionalism, cheap, and safe. Nevertheless, smaller particle sizes (<1 mm) have proven to be more difficult to separate visually due to low confidence in color or shape identification [52]. This is one of the limitations with visual as well as the questionable error rate of up to 70% [53]; this is because suspected MPs can be other materials until confirmed by chemical analysis [14]. Visual analysis can be easily contaminated by impurities in the environment; hence, the accuracy is lower [54]. These limitations are justification for the need for more advanced instrumental techniques for MP analysis. Hence, it could be said that visual analysis in MP assessment is a pre-step to more sophisticated, accurate instrumental analysis. Both the naked eye and microscopic analysis can be classified as visual methods, though microscopic analysis is more efficient than the naked eye, the accuracy remains relatively low [54].
2.3. Microscopy
By focusing and directing a high-energy electron beam across a sample using electromagnetic fields, the electron microscopy method creates a high-resolution picture of the object [55]. This makes this non-invasive, non-destructive technique a great approach to MP identification as it provides a two-dimensional morphology of the particles. One of the disadvantages of this technique is that factors such as sample preparation, particle size, handler expertise, and resolution of the electron microscope can affect the detection level. Also, it is not a quantitative technique and will give no details on the chemical composition or concentration, which is important in many microplastic studies [50,54,56].
Notably, the use of fluorescent dye to dye MPs and observe under a fluorescence technique adds more accuracy in the visual method of MP analysis [57,58] and has been used in some research [9,27,29,58,59,60,61]. It is one of the easiest and most cost-effective microscopy techniques in MP analysis. Dyes such as Nile Red are commonly used in this technique to label the MP samples, thereby making the MPs visible when viewed under ultraviolet (UV) light [62,63]. This technique can detect particles at a low level and is non-invasive and highly sensitive [50]. However, fluorescent microscopy is disadvantageous when compared to the electron microscopy technique with regards to the image resolutions as the dyes can become attached to other impurities in the samples, thus generating false results, and reducing the reliability of the result [64]. In addition, false results can arise from bioorganic interference, similarity in the color of the dye, and some environmental particles [54]. Nevertheless, an efficient and excellent MP recovery rate can be achieved from this technique when the samples are left for an average of 20 min in the Nile Red dye [15,58]. It is worth noting that stained particles can be further analyzed using FTIR and Raman spectroscopy without reducing the accuracy of the results. This combinative approach was used in analyzing MP presence in wine [23] and in seafood [65,66]. Importantly, scientists must carefully select this technique and exercise caution in its usage.
Scanning electron microscopy (SEM) technique is a non-destructive analytical method in microplastic assessment. SEM is a distinguished type of electron microscope. This has more accuracy than basic optical microscopy as the resolution of SEM can reach as low as 1 nm. Electron beams interact with the samples, thereby producing a high-resolution image of the object’s surface regardless of the particle’s color [67]. Despite its higher magnification and better imaging, images from scanning electron microscope cannot be used in color and chemical composition analysis [50,54,56] and this electron beam can also have a detrimental effect on the sample, thereby affecting the result [68]. In addition, transmission electron microscopy such as SEM can do better than an optical microscope in observing fine particles down to <0.2 μm, as seen in Gigault et al. [60] when dynamic light scattering, and electron spectroscopy were used on plastic particles in water.
Using SEM as a visual technique offers a perfect magnified image of the MP samples. It is usually attached to the energy dispersive X-ray spectroscopy (EDS) component for better efficiency. EDS is an important tool in a detailed analysis of types and elemental constituents in microparticles [69]. When combined with SEM as SEM-EDS, this technique can analyze MP surface morphology as well as elemental and chemical constituents synchronously which makes it a stronger tool than using only the electron microscope [53,70,71,72]. This combination of microscopy and spectroscopy enhances MP identification by analyzing the elemental composition of the plastic particles using an electron beam that scans the sample surface, and, as a result, generates a high-resolution three-dimensional image that allows for proper identification of the MP types present in the sample [73]. It offers more than SEM by providing data on the size, constituents, shape, and distribution of MPs in any environmental sample. For example, Tiwari et al., Zhang et al. and Liu et al. have used this combination technique in analyzing MPs [31,49,73]. SEM properly analyzes the elemental composition of MPs which are used in identifying carbon-presiding plastic polymers to avoid interfering impurities [74]. In Tiwari et al. [73], 40 chemical and morphological characterizations of plastic particles were carried out using SEM-EDS. Information on the age and weathering status of the plastic polymers identified in the samples was postulated after using this technique. Nevertheless, there are still some demerits of this combinative tool [54]. One of the requirements of SEM-EDS usage is that the sample particle should be conductive; however, most MPs are non-conductive. Though this is easily solved with a pretreatment process such as gold plating as discovered by Fu et al., it is a tedious and time-consuming pretreatment solution to do gold-plating of the sample before MP analysis is carried out [75]. This is not feasible for a large sample size as it may take days to complete the analysis. Also, other technical errors could affect the efficiency of the analysis of MP using the SEM-EDS technique [56]. It is used to analyze specific microplastic types; hence, this combinative tool is therefore taking unnecessary time and cost inefficiency. In addition, SEM or SEM-EDS cannot be used in research where the detailed composition of the MP particles needs to be characterized, as it is not one of the best quantitative techniques but can be combined with FTIR [76,77].
2.4. Spectroscopy
This instrumentation analysis is one of the most reliable identification methods in microplastic assessment due to the ability to record the specificity of the chemical bonds contained in the polymer using comparison [18]. As seen on Figure 2, there are other spectroscopic techniques, but FTIR is the most common analytical instrument in quantitative and chemical analysis of MP [78,79,80,81,82,83]. FTIR follows the principle where the samples absorb infrared light as a function of the wavenumber. This non-destructive technique accurately identifies the characteristics of MP particles through IR spectra observation, which detects the color or texture that is specific to them, reduces the chances of false results, and increases detection [13,26]. Though Raman spectroscopy follows the process of irradiating the particle with a laser beam that generates a specific spectrum for each polymer following the differences in the scattered light frequency works similarly to FTIR, analyzes each sample, and provides detailed composition data on them [13,84].
In chemical component analysis of MP using FTIR, infrared spectra are produced from the changes in bond dipole moment [43]. This concept heavily relies on spectral mapping and recording which have been documented in a library. The spectrums of the polymer identified through vibrational bands are compared with the spectrum database [23], the sample can be irradiated within IR light of 400–4000 cm−1 wavelength. FTIR offers more than to ascertain the presence of plastics but also identifies the polymer type by the type of chemical bond present. FTIR has been successfully used in the identification of carbon-based polymers [38], which include plastic types such as polyethylene (PE), polyamide (PA), polypropylene (PP), polyethylene terephthalate (PET), polyurethane (PU), polystyrene (PS), polyvinyl chloride (PVC), and polyester (PES).
Modes of FTIR include specular reflection, transmission, and attenuated total reflection [85], the type and characteristics of the sample determine the choice of the mode of FTIR technique to use. These are optimized technologies such as micro-FTIR, ATR-FTIR (Attenuated Total Reflection–Fourier Transform Infra-red), and micro-Raman spectroscopy. ATR-FTIR provides very high-quality imaging spectra with matching high accuracy; a specular reflection with the least accuracy, though, is suitable for analyzing MP in real samples [54,86]. The best technique, ATR-FTIR, also produces less noise as it has more resistance to interference from contaminants. It does not really need pretreatment unlike the transmission mode, which is more susceptible to impurities effects, has an efficiency reduction when sample thickness is <5 µm, and specular reflection that has a low matching degree with mapping, low accuracy, weak signal, and noise interference. However, this mode is expensive and not a perfect choice for large-scale MP analysis [43,54].
They are more efficient as MP analysis is more microscopic and micro spectrometry because of the micro size of the plastic particles. They have been used in soil MPs assessments [3,14,15,24,27,87]. RS outperforms FTIR in wet sample analysis and generates spatial chemical MP images of even extremely small-sized plastic particles. In a review article by Cabernard et al. [88], micro-Raman spectrometry comparatively performed 23% more than FTIR in MP quantification in the aquatic samples. As shown in Table 3, this technique catches impurities from color and light interference caused by contamination on MPs [74]. Another drawback of this spectroscopic method is the difficulty in applying it to ultra-fine plastic particles. More importantly, FTIR usage in assessing microplastic in soil has a very high efficiency rate because it is less susceptible to interference from other non-plastic substances in the sample. This efficiency depends on how well the organic matter (OM) is removed from the sample using easy pretreatment. This is important because soil and compost matrices are highly heterogenous and especially rich in OM and minerals [89,90]. FTIR is a non-destructive analytical technique and is non-invasive [89,91,92,93]. In addition, FTIR is environmentally friendly [94]. Though it is the most common instrument used in MP analysis so far, FTIR is disadvantaged in being restricted to dry samples only, unlike RS which can analyze both wet and dry samples without interference with the result. Raman spectroscopy is also advantageous because the samples are preserved for continuous studies, it is fast, non-destructive, highly sensitive, requires little sample, and is environmentally friendly. Despite these merits, RS is highly disadvantaged regarding cost-effectiveness. However, it is recommended for MP studies, especially with samples from agricultural matrices such as organic compost, sewage sludge, and soil, since wetness has no interference with the analysis. There are three major ways to read the vibration spectra data in a spectroscopic analysis, either by manually identifying the characteristics of the vibration bands or by automated use of a database containing library reference [6], which the spectrum is compared with; alternatively, this can be read using artificial intelligence [13,95].
Table 3. Advantages and disadvantages of various microplastics analytical options.
| Technique | Analyzable Sample Size | Cost | Time | Advantage | Disadvantage |
|---|---|---|---|---|---|
| Visual (naked eye) | Large particle size | Cheap | Fast | Fast and easy technique to use | Inability to verify polymer structure. Higher chance of misidentification. Unable to detect particles < 100 μm). |
| Microscopy, SEM | Down to micron (μm) range | Less expensive, Expensive | Fast (Optical microscope), Less fast (SEM) |
Easy identification of physical features. The abundance of MPs can also be carried out using this technique. It is non-destructive. | Cannot determine composition. Might need additional technological software to increase efficiency, e.g., SEM-EDS. Requires pretreatment, especially for non-conductive MPs |
| FTIR | Larger particle size can be analyzed from >500 mm by ATR-FTIR while micro-FTIR (μ-FTIR) can analyze as low as 20 mm | Expensive | Less fast | FTIR (μ-FTIR, ATR-FTIR, and focal plane array FTIR (FPA-FTIR)) allows a great detection limit of MPs to 5–10 μm. FPA-FTIR can swiftly and automatically scan sample filters to obtain spectral information and provide images. Detailed analysis of identification, quantification, and characterization. Has a comprehensive polymer library. Non-invasive. Non-destructive. |
Ineffectively analyze wet samples. Refractive error causes unexplained spectra from reading shape irregularities of MPs. Takes time and expertise to operate. The probe makes contact and pressures the sample particles and can damage them in the process, leading to loss of MP. Requires pretreatment to reduce spectral error or noise. Weathered plastic particles increase interference. |
| Raman Spectrometry | When coupled with microscopy method, Raman spectrometry method can analyze up to >1 μm plastic particle size | Expensive | Less fast | Efficient in detecting particles < 1 μm and the spatial resolution < 1 μm and even 500 nm sometimes. Analyzes both dry and wet samples, and simultaneously identifies pigments. Can be used for chemical mapping. Spectral unaffected by UV degradation, and shape of sample. |
Organic/inorganic contaminations can cause interference with fluorescence that affects spectra and identification. It is time consuming. Automatic mapping by μ-Raman spectrometry is still being developed. Requires pretreatment for increased efficiency and removal of impurities. |
| Mass Spectrometry (Pyr/GC/MS, TGA-GC-MS, TED-GC-MS) | All particle size | Expensive | Less fast | Usually does not require pretreatment thermal removes impurities including OM before analyzing the sample. No limitation, MP particle size is manually placed into the pyrolysis tube. GC/MS have several mass spectral libraries especially if using electron ionization. More detailed information on the components in the particle sample. Ability to distinguish polymers from additives. |
Sample must be volatile before GC/MS analysis. MS requires highly skilled personnel to run analysis to finish. Cannot simultaneously analyze multiple particles. Destructive, leads to loss of sample. Unable to obtain the robust data of samples being analyzed as they lost. |
| Hyperspectral Imaging | Might be unable to detect MP particles of less than 100 μm | fewer expensive | Very fast | Visual selection of the removed samples. Little sample pretreatment required. Cheaper than FTIR and Raman. Analyzes large sample size. | Large redundant data Requires complex data analysis. No standardized spectral matching model, still being developed. |
Following a combinative approach of analysis, RS and chemical digestion were used to identify polyethylene among other MP types of <10 µm from an effluent [96]. Araujo et al. [94] also reviewed this technique as better, faster, and stronger for identifying MP particles in environmental samples of <20 µm as used in some research studies. Also, FTIR and µFTIR have been used several times in both terrestrial and aquatic MP studies [49,86,97].
Organic matter, as well as the wetness of the sample, is disadvantageous to the efficiency of the spectra reading because it alters the polymer signals, and thus the magnitude can be misread, and a wrong identification made. This is a bigger concern in the compost where there is a higher concentration of OM; however, pretreatment of the sample with the most effective and least reactive, Fenton’s reagent [27], hydrogen peroxide is efficient in removing interfering OM from the sample before further analysis is carried out as shown in Table 2 and Table 3. There are other pretreatments required such as ultrasound, acidic/alkaline, density and electrostatic separation, and oxidizing treatment. The combination of all or more than two of these might increase the efficiency of the MP analysis. It is worthy of note that despite the efficiency of micro-spectroscopy in identification and quantification, they are time consuming and provide data on a small sample size, difficult to analyze samples of certain colors [76]. Fourier transform infrared spectroscopy still needs more technological advancement as it can only identify dry samples that have >20 µm since the spatial resolution of FTIR spectrum is of range 10–20 µm [43]. FTIR is also susceptible to ranging factors, which include the age of MP, heterogeneity of MPs, and other environmental impurities [54]. Depending on the purpose of the project and the turnaround time, this may not be the best choice, especially with an assessment of MPs on agricultural fields and compost facilities.
Other fast or advanced instrumental techniques (Figure 2) in MP assessment that need no or little pretreatment or are restricted by particle sample size include near-infrared (NIR) spectroscopy, HSI, and NMR. NIR, which requires no pretreatment, has a detection size of >1 μm and can predict the concentration of MPs [22,23,54,98,99,100].
Nuclear magnetic resonance (NMR) spectroscopy is non-invasive, non-destructive, fast, has high accuracy, is prone to low error, and can analyze plastic particles qualitatively and quantitatively [5]. NMR gives information on the molecular dynamics as well as the interactions in a molecule. NMR provide accurate three-dimensional (3D) structural data from vibrations from molecules within the sample environment, without destroying the sample. In addition, quantitative NMR (qNMR) has a high tolerance to environmental or impurities interference. Though these characteristics make them advantageous, it is worth noting that NMR spectroscopy is not cost efficient, though it can be cheaper when the characterization of polymers by NMR uses low-field instruments [5,23,44]. While sample preparation is not required, deuterated solvents such as chloroform (CDCl3, CHCl3) and trifluoroacetic acid (TFA) [5] are mostly required to use this technique. In addition, NMR has low sensitivity, especially when there are weak molecular vibrations and interactions. Also, the technique does not support the analysis of a higher molecular weight due to difficulty in reading the spectra [5]. High-resolution (1H NMR) spectroscopy and qNMR techniques usage are recently increasing in MP studies involving PE, PET, PS, PVC, and PA [5,44].
NIR spectroscopy is not so successful yet in solving the analytical curiosity of MPs in soil, and the hyperspectral imaging (HSI) technique is effective in the field as it works for soil surface scanning only [68,101]. This is because this instrument was originally designed for remote sensing of the earth’s surface; however, as shown in (Figure 3), HSI has been introduced into plastic waste studies through the recycling industry [28,101]. Based on a similar principle as the red, green, blue image, hyperspectral imaging instruments employ dispersive elements coupled with sensor arrays that allow a wider coverage area in less time than FTIR instruments [6]. HSI, which was first utilized in MP analysis by Karlsson et al. [102], removes selection bias. After sample preparation and pretreatment, the samples can be transferred manually into a Petri dish or filtered on glass fiber filters to be imaged by the hyperspectral camera (Figure 3). Smaller sample sizes are filtered, while particles > 500 µm can be manually moved onto the Petri dish [6,78]. The output for the HSI tool is also a spectrum for each pixel. These spectra are then subjected to data analysis to identify chemical signatures and give data on the MP particles, including the polymer type, size, number, and shape [6,101,103,104] (Figure 3).
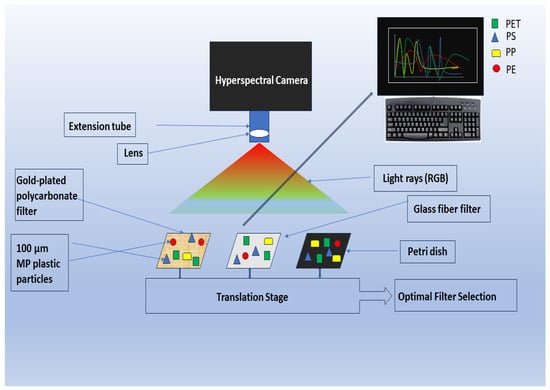
Figure 3. Framework of hyperspectral imaging for MP analysis.
One similarity between HSI and FTIR is that the two techniques produce the same data based on spectra in IR; however, the analysis time and data depth differentiate both instruments. At the cost of a longer time of analysis, a more detailed spectrum is given when FTIR is used while the HSI spectrum gives less detailed information, but the imaging of large areas can be covered in minutes. Serranti et al. [105] analyzed over 700 particles in 17 images, though the exact time was not reported. However, HSI has been reported to analyze an image area of 30 × 20 mm in 30 s [32], and about 1 min for a 120 × 120 mm image area [102]. FTIR spectra cover a wavelength range of 2500–25,000 nm, while HSI usually focuses on a smaller range of visible light (400–700 nm) and this limits the spectrum details, but the IR region of 1700–1800 nm captures the characteristics of C-H bond which can be used to identify organic molecules, while the wider spectral range for FTIR allows for information on various chemical bonds including distinct peaks for functional groups [6]. Another similarity between Raman, FTIR, and HSI is that the pretreatment of samples removes interference during the analysis of the plastic particles [32,104]. HSI as an instrument could be used in analyzing plastics extracted from soil samples. To do this, density separation and sample treatment will be carried out before analysis [32]. In previous studies, polymer type (PE, PS, PP, PET), particle size, and morphology have been identified using HSI [6,32,104,105]. In addition, the cost of an HSI instrument is expensive, though this depends on the type of system. However, FTIR and Raman have been reported to cost more than the HSI tools [106]. Though short analysis run time is a core strength of the HSI technique, a non-standardized spectral library is a disadvantage [32]. Over the years, some spectral matching techniques including plastic mapper (PlaMAPP), spectra angle mapper (SAM), and partial least squares discriminant analysis (PLS-DA) have been developed and used in some MP studies [6,32,98,101,102,105,107,108]. Another advantage of FTIR and Raman over HSI is that when using FTIR and Raman instruments, the particle recognition software reduces bias, but the analysis takes longer time to run as they are carried out individually unlike with HSI instruments. However, combining FPA with FTIR makes it run similarly to HSI because the analysis time then is dependent on the area and not particle number. Nonetheless, HSI remains a faster technique [6].
2.5. Thermal Techniques
Another alternative to overcoming the issue of OM interference in spectroscopic analysis in MP assessment in soil and compost matrices is through thermal analytic technology, such as Pyr/GC/MS [82,109,110,111,112], and thermal extraction desorption gas chromatography–mass spectrometry (TED/GC/MS) [8,23,67,69,113]. Mass-based techniques are faster than FTIR and Raman, which could take days to analyze samples depending on the particle number, however, there is a loss of specific data on the particle size especially with the thermo-analytical technologies [5]. Another advantage is that the concentrations of the MP particles can be measured in milligrams or grams of the sample. This properly measured specification enables easy comparison of results with other analyses and can be useful in the standardization of techniques, methods, and results. Since MPs can act as vectors by allowing the adsorption of chemical contaminants from the environment, thermal analytic technologies offer a direct, simple, and accurate MP contamination concentration analysis [65,70,111,114]. Analysis of adsorbed chemicals onto MPs can be stressful and challenging [115], but necessary for environmental sustainability and human health [114].
However, this is a destructive analytical method, as the sample collected from the interested environmental matrix is completely pyrolyzed before being passed into the gas chromatography instrument for analysis. In the process of changing the state of the sample, some information is lost [82]. Though not affected by OM presence, MP characterization such as shape, color, and size cannot be known using this analytical method. Hence, if identifying such data is an objective in the research, then Pyr/GC/MS is not recommended. Another disadvantage is that this process cannot synchronously analyze different plastic particles, but only one MP type [67]. Though no extraction process is involved, and this saves time, it is difficult to explain what happened to the impurities contained in the sample before pyrolysis took place. These impurities can create noise in the spectral readings and be mistaken for an actual reading of concerned chemicals. Pyr/GC/MS instrument is not commonly applied in soil MPs analysis as the physical counting of plastic particles is an important step in MPs analysis [9]. Pretreating the sample of OM interference, though possible, will be tedious and negate the simplicity, time saving, and ease of chromatography spectroscopic analysis. Also, there can be a risk of plastic fragmentation/degradation, as well as contamination if this is carried out [116,117]. Another thermal technology in MP analysis is thermal extraction desorption gas chromatography–mass spectrometry (TED/GC/MS), which has been shown to be efficient in detecting impurities and polymer types [111].
The thermogravimetric analysis (TGA) technique analyzes the mass loss of a plastic particle based on its degradation as a function of time or temperature. It is correlated with temperature because various plastic types have their distinct thermal degradation profile, and this degradation is directly related to the constant mass loss as a function of time [31,67]. Some of the advantages of the TGA technique include its relatively low cost, no requirement of sample pretreatment, and simplicity to carry out [65]. Nevertheless, this technique will not allow for the continuation of studies on the same sample as it is destructive. A methodology based on both TGA analysis and statistical analysis called the Universal Soil Model (SUMM) was established by David et al. [65] for the qualitative and quantitative identification of the most frequent MPs, such as PE, PS, PVC, and PET, in agricultural soil samples. Compared to the Py-GC-MS approach, the TGA analysis has a significant benefit since it uses a relatively high number of samples, which enables representative findings.
MP studies have progressed based on combinative instrumentation analysis. This can be seen in Table 1, where some researchers combined two or more instruments for assessing the quantitative and qualitative data of MPs [23,25,28,81,93].
3. Microplastics Legislation
The more preventive approach to the management of microplastic pollution in the environment is to enact laws and regulations that will help mitigate against the abundance of MPs in both the terrestrial and aquatic environments. Legislation is an important instrument to address MP pollution at all levels, including local, regional, national, and international [118]. This is because as emerging pollutants, there are several uncertainties about MPs, especially the sources, distribution, fate, bioavailability, and toxicity to plants, animals, and humans [119,120,121,122]; however, for the same knowledge paucity, it is difficult to develop suitable legislation. Legislation cannot be independent of knowledge and can also strengthen awareness [30,123]. Over the/ past three decades, legislation has been developed to properly reduce the risks, impacts, and management of plastics, according to the three categories of larger plastics, medium plastics, and smaller plastics. However, for smaller plastics, where MPs belong, the focus has been more on preventing microbeads in aquatic environments.
MPs are categorized into two types, the primary MPs, and the secondary MPs. Primary MPs are more prevalent in aquatic environments as they are plastic particles that are deliberately created. They are usually found in wash-off household and industrial cleaning products, toothpaste, and cosmetics [119]. Secondary MPs are plastic particles from the fragmentation of larger plastics over a long period of time. This MP type is abundant in the terrestrial environment as leftovers from microplastic waste. It is reported that the United Nations Environmental Program (UNEP) stated that the number of microbeads in an exfoliating gel could almost be the same number of plastics used in packaging the product [124]. With microbeads abundant in the water, aquatic animals can ingest these plastics, which can threaten their lives, and potentially human health when taken up through the food chain, as MPs have been found to have the capability to translocate into the circulatory system and accumulate in human lungs, liver, and brain [80,119,125,126,127]. This information on microbeads prompted some countries to enact laws to prohibit microbeads in cosmetic and cleaning products. The United States federal government established the Microbead-free Water Act of 2015 which became active in 2018, while the Canadian parliament passed legislation that prohibited the manufacture of microbeads in 2017, which brought a ban in 2019. In addition, the Australian Working Group, and the Council of the European Union joined in the fight against microbeads in the aquatic environments through legislation [119,128,129]. More of these countries and their laws are represented in Table 4.
Table 4. Some national and organizational legislation of microplastics.
| Country/Organization | Policy/Legislation | Plastic Category | Aim |
|---|---|---|---|
| United States | Microbead-free Waters Act 2015 | Aquatic MPs | Ban—production and sales of wash-off cosmetic products |
| The Break Free from Plastic Pollution Act 2023 | Plastics | To shift financial responsibility of plastic waste management to producers of plastics. Ban single use of plastic products. Prohibit export of plastic waste. |
|
| France | Circular Economy Law (Waste Prevention and Management) 2018 (modified—2020) | Aquatic MPs | Ban cosmetics products containing plastic particles. |
| Draft Law on Combating Plastic Pollution (adopted 2022) | Microfibres, microbeads | To regulate loss and leakage of industrial granules, prohibit intentional usage of microbeads in detergent, and provide impact assessment on textile industry of plastic fibers | |
| European Union | The Packaging and Packaging Waste Directive (Plastic tax) | Reduce plastic waste | |
| Canada | Microbeads in Toiletries Regulations (2017) | Aquatic MPs | Reduce the amount of plastic microbeads entering Canadian freshwater and marine environments. |
| Single-use Plastics Prohibition Regulations (2022) | Larger plastics | To prohibit manufacture, importation, and distribution of single-use plastic products | |
| Kenya | Plastic Bag Control and Management Regulations (2018) The Wildlife Conservation and Management Act 2020 |
Larger plastics | Reduce usage, manufacture, and importation of plastic bags. Ban on single-use plastic products. |
| Australia | The Plastic Reduction and Circular Economy Act 2021 | Aquatic MPs | Ban—distribution of wash-off personal care product |
| New Zealand | Waste Minimization Act through Waste Minimization (Microbeads) Regulations 2017 | Aquatic MPs | Prohibited plastic beads as an ingredient in personal care products |
| United Kingdom | Environmental Permitting Regulations 2018 | Aquatic MPs | Banned cosmetics and cleaning products containing microbeads. Charge levies on single-use carrier bags Ban single-use plastics |
| Larger plastics | |||
| Northern Ireland | The Environmental Protection (microbeads) (Northern Ireland) Regulations 2018 | Aquatic MPs | Prohibited the use of plastic beads |
| China | 2019 Industrial Catalogue | Aquatic MPs | Ban—production and sales of cosmetics containing microbeads |
| EU | The Single-use Plastics Directive 2019 | Aquatic MPs | Target eradicating 10 most common single-use plastics found on Europe’s beaches and seas |
| The Ocean CleanUp | Clean up | Aquatic plastics | Developing technologies to reduce plastics in ocean by 90% by 2040 |
| Thailand | Thailand Ministry of Public Health (2019) through Roadmap on Plastic Waste Management (2018—2030) | Aquatic MPs | Ban the production, sales, and distribution of cosmetics with microbeads as an ingredient. |
| Larger plastics | Ban single use of plastics. | ||
| World Wildlife (WWF) | Regulations | Larger plastics | Establish a globally legally binding agreement to end plastic pollution |
| The Netherlands | Environmental Management Act (The Commodities Act Decree) | Plastics waste | To control packaging and consumer products Regulate single-use plastic |
| Ireland | The Microbeads (Prohibition) Act 2019 | Aquatic MPs | Ban the use of plastic beads in households and industrial cleaning products |
| India | Plastic Waste Management (Amendment) 2022 | Larger plastics | Phase out single-use plastic |
| Germany | The Germane Ordinance on Single-use Plastics 2021 | Larger plastics | Reduce impact of plastic waste on the environment Ban some single-use plastic products |
| South Africa | The National Environmental Management Waste Act 2008 (amended 2014) through National Waste Management Strategy 2020 | Larger plastics | Reduce production of single-use plastics destroying marine environment |
| Wales, Ireland, Scotland | Tax/levies on single-use plastics | Larger plastics | Discourage the single use of plastic products to reduce waste |
| Berkeley, California | The Single-use Foodware and Litter Reduction Ordinance (2022) | Larger plastics | Reduce plastic waste in the environment |
| United States (15 States and territories) | Banned disposable plastic bags | Larger plastics | Reduction of plastic waste |
While there are legislations addressing microbeads in water bodies, this is insufficient as there are other forms of MPs in the environment. The legislation on larger plastics is a step in controlling and managing plastic wastes; however, there is a need for more since MPs can act as vectors, adsorbing microorganisms, heavy metals, and persistent organic pollutants [125]. A study showed that globally, an average of 5 g of plastics is being ingested by people on a weekly basis [130]. As shown in Table 4, some countries have placed bans and restrictions on the importation and production of plastic and fabric products. This action could help with reducing the number of secondary MPs in the terrestrial environment, including agricultural soil. MPs access the agricultural environment through the application of soil enhancers (biosolids, compost), mulch films, fragmentation of plastic packaging, irrigation, seed coating, and agrochemical encapsulates [40,58,131]. Bans and levies have been employed in controlling plastic pollution over the years. In 1991, Germany implemented a policy to reduce plastic bag consumption [119,132], and Bangladesh and more than 60 countries implemented a ban on low-density polyethylene (LDPE) [119,133].
There is a need for more scientific research on MPs in the soil (terrestrial) environment as the progress reports will continually form the basis for improvement of regulations in restricting the increase in plastic pollution [118]. In addition, the fight against plastic pollution will be easier with the collaboration of every nation, organizations (including the plastic industry), and individuals. A month after the enforcement date of the Single-use Plastics Prohibition regulations, a group in Ontario, Canada successfully overturned the ban for single-use plastics (checkout bags, straws, cutlery, food packaging, and stir sticks) [134]. The court ruled against the ban, stating the prohibition of the plastics was “unreasonable” and “unconstitutional”. Also, while European states are jointly working to eradicate deliberate plastic waste in the environment [135], the United States only has about a third of the countries that are actively fighting against plastic pollution [136]. It is still early to evaluate the effect of the legislations and regulations in Table 4 [133], as they are only recently established, and have yet to be enforced in Canada; yet it is important to unite to achieve the desired goal of reduced plastics in the environment.
This entry is adapted from the peer-reviewed paper 10.3390/microplastics3010001
This entry is offline, you can click here to edit this entry!
