Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Avian influenza is caused by avian influenza virus infection; the H5N1 avian influenza virus is a highly pathogenic subtype, affecting poultry and human health. Since the discovery of the highly pathogenic subtype of the H5N1 avian influenza virus, it has caused enormous losses to the poultry farming industry. It was recently found that the H5N1 avian influenza virus tends to spread among mammals. Therefore, early rapid detection methods are highly significant for effectively preventing the spread of H5N1.
- H5N1 subtype
- avian influenza virus
- detection technology
1. Introduction
Avian influenza (AI) is an infectious disease caused by avian influenza virus (AIV) infection; it exhibits various symptoms, ranging from effects on the respiratory system to severe systemic sepsis. According to two surface glycoproteins of AIV, the antigens of hemagglutinin (HA) and neuraminidase (NA) can be divided into different subtypes; so far, 16 HA subtypes (H1–H16) and 9 NA subtypes (N1–N9) have been identified [1]. AIV can infect and reproduce in macrophages and lymphocytes, leading to the destruction of lymphocytes [2] and causing a series of respiratory and gastrointestinal symptoms. In mid-February 2009, a swine flu outbreak (H1N1 influenza virus) broke out in Mexico, causing a widespread epidemic, but the symptoms of most infected individuals were mild [3]. H5N1 AIV can be transmitted from birds to humans, and the mortality rate is extremely high [4]. Since 2003, 17 countries around the world have reported 861 cases of human infection with H5N1 avian influenza to the World Health Organization (WHO). In addition, most cases have occurred in Asian countries, and the mortality rate of all cases exceeds 50% [5].
In 1996, H5N1 AIV was initially isolated from geese in Guangdong province [6], and human cases of H5N1 AIV infection were reported in 1997 in a poultry outbreak in Hong Kong, China. Since then, H5N1 AIV has continued to spread among poultry populations and has had a serious impact on the global poultry farming industry [7]. Since 2003, H5N1 AIV has dominated poultry in southern China and Southeast Asia [8]. With the global contagion of H5N1 AIV, which has had a growing impact on wild birds [9], in the summer of 2021, an outbreak of H5N1 avian influenza in several populations in Scotland resulted in the deaths of many breeding Great Skuas (Stercorarius skua) [10]. The seasonal migration of Stercorarius skua may lead to its spread to other areas. In December 2021, poultry deaths occurred on a farm in St. John’s, Canada, due to infection with H5N1 avian influenza [11]. In January 2022, on the west coast of Namibia, many double-crested cormorants (Phalacrocorax auritus) died on account of infection with H5N1 avian influenza [12]. On January 9, 2023, the World Health Organization received a report that Ecuadorians were infected with H5N1 avian influenza [13]. The years 2014–2015 and 2022–2023 represented two major disasters for poultry. The dominant subtype is H5N1, so it is highly important to study the detection technology used to detect H5N1 AIV.
2. Surface Plasmon Resonance (SPR)
Surface plasma technology was introduced to the sensor boundary in the early 1980s; it can be described as a collective oscillator in free-electron plasma at the metal boundary [14]. SPR biosensors are mainly composed of three components: an optical waveguide device, a metal film, and a biomolecular film [15]. The detection principle of SPR is identified in Figure 1. Bai et al. [16] used biotinylated DNA aptamers as specific recognition elements for portable SPR biosensors, and then fixed them on the gold surface of the sensor coated with streptavidin through a combination of streptavidin and biotin. Fixed aptamers capture the H5N1 avian influenza virus, increasing the refractive index (RI). The sensor was able to detect H5N1 AIV with a concentration of 0.128–12.8 HAU within 1.5 h. Wong et al. [17] established an SPR biosensor for the detection of H5N1 AIV antibody biomarkers, which requires neither time-consuming interference fringe analysis nor any extraction process; it is unlabeled and works in real-time. In general, SPR biosensors have the advantages of being label-free, highly sensitive, and high throughput. However, because SPR is based on the measurement of reflected light, if the tested sample is Turbidite, the detection result will be affected.
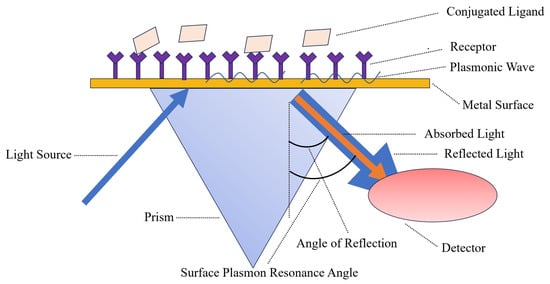
Figure 1. The light source of the SPR biosensor is polarized light, and the sensing chip is coated with a layer of gold film. In the experiment, a target molecule is fixed on the surface of the gold film, and then the molecules that interact with it are dissolved in the solution and flow through the chip surface, causing the SPR angle to change accordingly.
3. Field-Effect Transistor (FET)
FET is a semiconductor device that utilizes electric field effects to control output current [18], with high input resistance (107–1015 Ω), low noise, large dynamic range, low power consumption, wide safe working area, and easy integration [19]. Gao et al. [20] established a novel semiconductor silicon nanowire FET (SiNW FET) biosensor that can detect viral nucleic acid in real-time; it can detect DNA as low as 1fM. Moreover, it has high specificity for single-base errors and can selectively detect H1N1 and H5N1 AIV at the same time. Guo et al. [21] fabricated indium tin oxide thin film transistors on glass substrates. Through 3-glycidyl oxygen propyl, silane will increase the oxygen radicals of the H5N1 avian flu virus monoclonal antibodies, which are fixed on the ITO channel; the introduction of the H5N1 virus affects the ITO TFT’s electronic properties, which generates the threshold voltage and leads to the change in the field-effect mobility. Kwon et al. [22] established a labeled FET-free biosensor for the detection of AIV in chicken serum, in which DNA aptamers are used as receptors for hemagglutinin proteins immobilized on gold microelectrodes. The specific binding of target proteins leads to changes in surface potential, resulting in a signal response of field-effect transistor transducers. In the range of 10 pM to 10 nM, it increases linearly with the logarithmic concentration of HA protein, with a detection limit of 5.9 pM. Sensors based on field-effect transistors have shown high selectivity and stability in H5N1 AIV sensing and are rapidly developing towards more sensitive, miniaturized, simpler, and more portable models [23]. However, the sensitivity and accuracy of field-effect biosensors still need to be improved, and the requirements for operators are relatively high.
4. Electrochemical Biosensor
Electrochemical biosensors are sensors based on the electrochemical characteristics of the object being measured; It converts the chemical quantity of the object to be tested into electrical quantities for sensing and detection. It is composed of a biological recognition body and an electrochemical transducing system, in which the biological recognition body has the function of specifically identifying various tested substances, and the transducing system can convert biochemical signals into electrical signals [24][25]. Kukol et al. [26] investigated a label-free detection system based on electrochemical impedance spectroscopy; it is capable of detecting the DNA sequence of an H5N1 AIV hybridized to a single-stranded DNA oligonucleotide probe linked to a gold surface. This method is simpler to perform than traditional EIS and is also able to analyze environmental samples for the presence of AIV. Liu et al. [27] developed an electrochemical biosensor for detecting the genetic sequence of H5N1 AIV with DNA aptamer immobilized on electrodes modified with mixed nanomaterials, using multi-walled carbon nanotubes (MWNT), polypyrrole (PPy) nanowires (PPNWs), and gold nanoparticles (GNPs) to assemble and modify the electrode to improve its selectivity and sensitivity. Shi et al. [28] successfully constructed a label-free electrochemical biosensor for the detection of H5N1 AIV and prepared a highly directed hybrid microarray on a gold substrate with good specificity and stability. Jarocka et al. [29] built a device based on a glassy carbon electrode electrochemical immunosensor; using a protein, they achieved resistance to its monoclonal antibody, recombinant His6 H5 HA antigen, and BSA-modified glassy carbon electrodes. Not only can it be used for the determination of the existence of the H5 monoclonal antibody in the blood in the buffer, but it also can be used to detect serum antibodies against H5 HA chicken. Diba et al. [30] established a sandwich detection platform containing surface-formed aptamer–protein–antibody complexes by introducing a new DNA aptamer/H5N1 protein/ALP-labeled anti-H5N1 sandwich complex on a gold-nanoparticle-modified carbon electrode. The amperometric detection of H5N1 AIV proteins can be achieved with high selectivity and sensitivity. Fang et al. [31] developed an unlabeled electrochemical sensor, which uses the H5N1 avian influenza virus gene sequence as the target DNA and [Fe (CN) 6]3−/4− solution as an electrochemical indicator. DNA aptamers were self-assembled on flower-shaped VS2, graphene, and Au nanoparticle-modified glassy carbon electrodes. Has good stability and repeatability. Fu et al. [32] developed an electrochemical resistance biosensor based on carbon nanotubes to detect H5N1 AIV. Using nitrogen-doped multi-walled carbon nanotubes (N-MWCNTs) and semiconducting single-walled carbon nanotubes (sc-SWCNTs) as active sensing elements, this biosensor is small, flexible, and easy to use. In general, the electrochemical biosensor has the advantages of strong specificity, good stability, and repeatability, but there are testing problems such as high costs and short lifespans.
5. Gene Biosensor
The principle of a gene biosensor is to hybridize the probe fixed on the surface of the sensor or transducer probe with another complementary ssDNA molecule; the double-stranded DNA will show a certain physical signal, which will finally be reflected by the transducer [33]. Lee et al. [34] developed a label-free biosensor for DNA hybridization detection that was not affected by small changes in the reference voltage; they successfully achieved the specific detection of the oligonucleotide sequence of H5N1 AIV. Grabowska et al. [35] developed a single-electrode genetic sensor capable of the simultaneous determination of the HA and NA sequences of H5N1 AIV, using two different oligonucleotide probes covalently fixed to a gold electrode surface through Au-S bonds. One probe was complementary to the cDNA of H5 with ferrocene modification at its 5′ end (SH-ssDNA-Fc), and the second probe was decorated with methylene blue at its 5′ end (Complementary cDNA of SH ssDNA MB and N1). This dual-gene sensor was selective and had similar detection limits for both genes. Grabowska et al. [36] established an electrochemical gene sensor with a reduction–oxidation (REDOX)-labeled oligonucleotide probe of metal carboborane [3-iron diterpene] on a gold electrode. The 5′ end of the probe was modified with an NH2 group and covalently attached to the electrode with 3-mercaptopropanoic acid SAM. The system is highly sensitive to targets containing sequences complementary to the probe and reacts very weakly to non-complementary targets. Malecka et al. [37] developed a genetic sensor for detecting specific DNA and RNA sequences of H5N1 AIV, achieving good selectivity. Malecka et al. [38] studied a gene based on a gold electrode electrochemical sensor, which exists in the sample solution of REDOX active markers [Fe (CN)6]3−/4−. Using a square-wave voltammetry analysis of signals, the gene sensor has good sensitivity and selectivity; what is more, it is easy to make. In general, genetic biosensors have good specificity and accuracy, but, at the same time, such biosensors are not suitable for field detection due to their complex design and significant environmental influence.
6. Impedance Biosensor
The impedance biosensor is placed into the corresponding detection medium, and a specific-frequency and small-amplitude AC potential wave is used to measure the ratio of the AC potential to the current signal, which is the impedance of the system and will change with the change in the measurement frequency [39]. The detection principle of impedance biosensors is displayed in Figure 2. Yan et al. [40] established an impedance biosensor based on interdigitated array microelectrodes (IDAMs) combined with immunomagnetic separation. Fix magnetic nanospheres coated with streptavidin onto biotin-labeled anti-H5 monoclonal antibodies, capture H5 AIV from the sample solution through specific immune reactions, and then separate and concentrate it using a magnetic field. From sampling to detection completion, impedance measurement of interdigital array microelectrodes in the frequency range of 20 Hz to 1 MHz can be completed in only 1.5 h. Lin et al. [41] designed and manufactured a separator based on a high-intensity, high-gradient magnetic field to improve previous portable impedance biosensor instruments, making them faster and more reliable. This improved impedance biosensor can recognize the H5N1 avian influenza virus within 30 min. Lum et al. [42] designed an improved impedance biosensor in which polyclonal antibodies of the N1 subtype are immobilized on the microelectrode surface and specifically bind to the H5N1 avian influenza virus. Chicken red blood cells are used as biomarkers for capturing the H5N1 avian influenza virus to amplify impedance signals. The improved impedance biosensors have higher specificity and reliability and shorter testing times. Lin et al. [43] developed a wet etching technology using the improved impedance of the sensor; the cost is lower than the dry etching process, and a new compact electrode design is used to increase microelectrode production and reduce the cost of the impedance biosensors. Lum et al. [44] selected DNA aptamers by phylogenetic evolution of index enriched ligands (SELEX) and immobilization of biotinylated aptamers targeting H5N1 AIV onto the electrode surface through biotin–streptavidin binding. The H5N1 avian influenza virus is captured on the surface of the microelectrode, causing an increase in impedance, and detection can be completed within 30 min.
Figure 2. Principles of impedance biosensors.
This entry is adapted from the peer-reviewed paper 10.3390/ijms242417157
References
- Fouchier, R.A.M.; Munster, V.; Wallensten, A.; Bestebroer, T.M.; Herfst, S.; Smith, D.; Rimmelzwaan, G.F.; Olsen, B.; Osterhaus, A.D.M.E. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J. Virol. 2005, 79, 2814–2822.
- Laudert, E.; Sivanandan, V.; Halvorson, D. Effect of an H5N1 avian influenza virus infection on the immune system of mallard ducks. Avian Dis. 1993, 37, 845–853.
- Neumann, G.; Noda, T.; Kawaoka, Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 2009, 459, 931–939.
- Patel, A.; Tran, K.; Gray, M.; Li, Y.; Ao, Z.J.; Yao, X.J.; Kobasa, D.; Kobinger, G.P. Evaluation of conserved and variable influenza antigens for immunization against different isolates of H5N1 viruses. Vaccine 2009, 27, 3083–3089.
- Chen, S.; Liao, J.; Wang, L.; Luo, P.; Guo, L.; Zheng, W.; Wu, Z.; Ying, Q. Rapid Detection of H5N1 Avian Influenza Virus Based on RAA Fluorescence Assay. China Port Sci. Technol. 2021, 3, 4–9.
- Xu, X.; Subbarao, K.; Cox, N.J.; Guo, Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: Similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 1999, 261, 15–19.
- Chen, H.; Smith GJ, D.; Li, K.S.; Wang, J.; Fan, X.H.; Rayner, J.M.; Vijaykrishna, D.; Zhang, J.X.; Zhang, L.J.; Guo, C.T.; et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: Implications for pandemic control. Proc. Natl. Acad. Sci. USA 2006, 103, 2845–2850.
- Smith, G.J.D.; Fan, X.H.; Wang, J.; Li, K.S.; Qin, K.; Zhang, J.X.; Vijaykrishna, D.; Cheung, C.L.; Huang, K.; Rayner, J.M.; et al. Emergence and predominance of an H5N1 influenza variant in China. Proc. Natl. Acad. Sci. USA 2006, 103, 16936–16941.
- Beyit, A.D.; Meki, I.K.; Barry, Y.; Haki, M.L.; El Ghassem, A.; Hamma, S.M.; Abdelwahab, N.; Doumbia, B.; Benane, H.A.; Daf, D.S.; et al. Avian influenza H5N1 in a great white pelican (Pelecanus onocrotalus), Mauritania 2022. Vet. Res. Commun. 2023, 1–5.
- Furness, R.W.; Gear, S.C.; Camphuysen, K.C.J.; Tyler, G.; de Silva, D.; Warren, C.J.; James, J.; Reid, S.M.; Banyard, A.C. Environmental Samples Test Negative for Avian Influenza Virus H5N1 Four Months after Mass Mortality at A Seabird Colony. Pathogens 2023, 12, 584.
- Caliendo, V.; Lewis, N.S.; Pohlmann, A.; Baillie, S.R.; Banyard, A.C.; Beer, M.; Brown, I.H.; Fouchier, R.A.M.; Hansen, R.D.E.; Lameris, T.K.; et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. Sci. Rep. 2022, 12, 11729.
- Molini, U.; Yabe, J.; Meki, I.K.; Ben Ali, H.O.A.; Settypalli, T.B.K.; Datta, S.; Coetzee, L.M.; Hamunyela, E.; Khaiseb, S.; Cattoli, G.; et al. Highly pathogenic avian influenza H5N1 virus outbreak among Cape cormorants (Phalacrocorax capensis) in Namibia, 2022. Emerg. Microbes Infect. 2023, 12, 2167610.
- Bruno, A.; Alfaro-Núñez, A.; de Mora, D.; Armas, R.; Olmedo, M.; Garcés, J.; Muñoz-López, G.; Garcia-Bereguiain, M.A. First case of human infection with highly pathogenic H5 avian influenza a virus in South America: A new zoonotic pandemic threat for 2023? J. Travel Med. 2023, 30, taad032.
- Nylander, C.; Liedberg, B.; Lind, T. Gas detection by means of surface plasmon resonance. Sens. Actuators 1982, 3, 79–88.
- Cai, Q.; Li, X.; Chen, Y. History, Current Status, and Prospects of Biosensors Based on Surface Plasmon Resonance. Foreign Med.—Biomed. Eng. Div. 1999, 2, 1–7.
- Bai, H.; Wang, R.; Hargis, B.; Lu, H.G.; Li, Y.B. A SPR aptasensor for detection of avian influenza virus H5N1. Sensors 2012, 12, 12506–12518.
- Wong, C.L.; Chua, M.; Mittman, H.; Choo, L.X.; Lim, H.Q.; Olivo, M. A phase-intensity surface plasmon resonance biosensor for avian influenza A (H5N1) detection. Sensors 2017, 17, 2363.
- Luo, X.; Xu, J.; Chen, H. Field effect transistor biosensor. Anal. Chem. 2004, 32, 1395–1400.
- Yang, J.; Zhang, Y.; Liu, C.; Li, J.; Xiao, Z.; Li, D.; Zhang, L.; Cao, Z. Studying and application of field effect transistor sensors. Chem. Sens. 2018, 38, 1–11.
- Gao, A.; Lu, N.; Dai, P.; Li, T.; Pei, H.; Gao, X.L.; Gong, Y.B.; Wang, Y.L.; Fan, C.H. Silicon-nanowire-based CMOS-compatible field-effect transistor nanosensors for ultrasensitive electrical detection of nucleic acids. Nano Lett. 2011, 11, 3974–3978.
- Guo, D.; Zhuo, M.; Zhang, X.; Xu, C.; Jiang, J.; Gao, F.; Wan, Q.; Li, Q.H.; Wang, T.H. Indium-tin-oxide thin film transistor biosensors for label-free detection of avian influenza virus H5N1. Anal. Chim. Acta 2013, 773, 83–88.
- Kwon, J.; Lee, Y.; Lee, T.; Ahn, J.H. Aptamer-based field-effect transistor for detection of avian influenza virus in chicken serum. Anal. Chem. 2020, 92, 5524–5531.
- Zhang, Z.; Chen, Y.; Song, L.; Su, Z.; Zhang, H. Research progress in the application of field-effect transistor biosensors in biomedical detection. China Biotechnol. 2021, 41, 73–88.
- Mo, J.; Zhou, X. Progress in the Development and Application of Bioelectrochemical Sensors. J. Shantou Univ. (Nat. Sci. Ed.) 1993, 1, 80–91.
- Zhang, Y.; Jiao, K.; Liu, C. Electrochemical Biosensor. J. Qingdao Inst. Chem. Technol. 1992, 2, 99–105.
- Kukol, A.; Li, P.; Estrela, P.; Ko-Ferrigno, P.; Migliorato, P. Label-free electrical detection of DNA hybridization for the example of influenza virus gene sequences. Anal. Biochem. 2008, 374, 143–153.
- Liu, X.; Cheng, Z.; Fan, H.; Ai, S.Y.; Han, R.X. Electrochemical detection of avian influenza virus H5N1 gene sequence using a DNA aptamer immobilized onto a hybrid nanomaterial-modified electrode. Electrochim. Acta 2011, 56, 6266–6270.
- Shi, L.; Chu, Z.; Dong, X.; Jin, W.Q.; Dempsey, E. A highly oriented hybrid microarray modified electrode fabricated by a template-free method for ultrasensitive electrochemical DNA recognition. Nanoscale 2013, 5, 10219–10225.
- Jarocka, U.; Sawicka, R.; Góra-Sochacka, A.; Sirko, A.; Zagórski-Ostoja, W.; Radecki, J.; Radecka, H. Electrochemical immunosensor for detection of antibodies against influenza A virus H5N1 in hen serum. Biosens. Bioelectron. 2014, 55, 301–306.
- Diba, F.S.; Kim, S.; Lee, H.J. Amperometric bioaffinity sensing platform for avian influenza virus proteins with aptamer modified gold nanoparticles on carbon chips. Biosens. Bioelectron. 2015, 72, 355–361.
- Fang, L.X.; Cao, J.T.; Huang, K.J. A sensitive electrochemical biosensor for specific DNA sequence detection based on flower-like VS2, graphene and Au nanoparticles signal amplification. J. Electroanal. Chem. 2015, 746, 1–8.
- Fu, Y.; Romay, V.; Liu, Y.; Ibarlucea, B.; Baraban, L.; Khavrus, V.; Oswald, S.; Bachmatiuk, A.; Ibrahim, I.; Rümmeli, M.; et al. Chemiresistive biosensors based on carbon nanotubes for label-free detection of DNA sequences derived from avian influenza virus H5N1. Sens. Actuators B Chem. 2017, 249, 691–699.
- Zhao, Z.; Cui, D. Gene Sensor. Electron. Eng. Prod. World 2000, 63–65.
- Lee, K.H.; Lee, J.O.; Choi, S.; Yoon, J.B.; Cho, G.H. A CMOS label-free DNA sensor using electrostatic induction of molecular charges. Biosens. Bioelectron. 2012, 31, 343–348.
- Grabowska, I.; Malecka, K.; Stachyra, A.; Góra-Sochacka, A.; Sirko, A.; Zagórski-Ostoja, W.; Radecka, H.; Radecki, J. Single electrode genosensor for simultaneous determination of sequences encoding hemagglutinin and neuraminidase of avian influenza virus type H5N1. Anal. Chem. 2013, 85, 10167–10173.
- Grabowska, I.; Stachyra, A.; Góra-Sochacka, A.; Sirko, A.; Olejniczak, A.B.; Lesnikowski, Z.J.; Radecki, J.; Radecka, H. DNA probe modified with 3-iron bis (dicarbollide) for electrochemical determination of DNA sequence of Avian Influenza Virus H5N1. Biosens. Bioelectron. 2014, 51, 170–176.
- Malecka, K.; Stachyra, A.; Góra-Sochacka, A.; Sirko, A.; Zagorski-Ostoja, W.; Dehaen, W.; Radecka, H.; Radecki, J. New redox-active layer create via epoxy–amine reaction–The base of genosensor for the detection of specific DNA and RNA sequences of avian influenza virus H5N1. Biosens. Bioelectron. 2015, 65, 427–434.
- Malecka, K.; Stachyra, A.; Góra-Sochacka, A.; Sirko, A.; Zagórski-Ostoja, W.; Radecka, H.; Radecki, J. Electrochemical genosensor based on disc and screen printed gold electrodes for detection of specific DNA and RNA sequences derived from Avian Influenza Virus H5N1. Sens. Actuators B Chem. 2016, 224, 290–297.
- Ye, Z. A Portable Impedance Biosensor Instrument for Rapid Detection of Chlorpyrifos and Escherichia coli. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2015.
- Yan, X.F.; Wang, M.H.; Wen, X.H.; An, D.; Yarlagadda, P.; Kim, Y.H. Rapid detection of avian influenza virus using immunomagnetic separation and impedance measurement. Appl. Mech. Mater. 2013, 239, 367–371.
- Lin, J.; Lum, J.; Wang, R.; Tung, S.; Hargis, B.; Li, Y.B.; Lu, H.G.; Berghman, L. A portable impedance biosensor instrument for rapid detection of avian influenza virus. In Proceedings of the SENSORS, 2010 IEEE, Waikoloa, HI, USA, 1–4 November 2010; pp. 1558–1563.
- Lum, J.; Wang, R.; Lassiter, K.; Srinivasan, B.; Abi-Ghanem, D.; Berghman, L.; Hargis, B.; Tung, S.; Lu, H.G.; Li, Y.B. Rapid detection of avian influenza H5N1 virus using impedance measurement of immuno-reaction coupled with RBC amplification. Biosens. Bioelectron. 2012, 38, 67–73.
- Lin, J.; Wang, R.; Jiao, P.; Li, Y.T.; Li, Y.B.; Liao, M.; Yu, Y.D.; Wang, M.H. An impedance immunosensor based on low-cost microelectrodes and specific monoclonal antibodies for rapid detection of avian influenza virus H5N1 in chicken swabs. Biosens. Bioelectron. 2015, 67, 546–552.
- Lum, J.; Wang, R.; Hargis, B.; Tung, S.; Bottje, W.; Lu, H.G.; Li, Y.B. An impedance aptasensor with microfluidic chips for specific detection of H5N1 avian influenza virus. Sensors 2015, 15, 18565–18578.
This entry is offline, you can click here to edit this entry!
