Anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma (ALCL) is an aggressive T-cell lymphoma characterized by large T-cells with strong CD30 and ALK expression. Although conventional chemotherapy is effective in most patients, approximately 30% experience a relapse or refractory disease and have a poor prognosis. Several risk factors associated with poor prognosis have been identified in pediatric ALK-positive ALCL. These include morphological patterns with the small cell variant or lymphohistiocytic variant, leukemic presentation, the presence of minimal disseminated disease, or involvement of the central nervous system. Relapsed or refractory ALK-positive ALCL is often resistant to conventional chemotherapy; therefore, salvage therapy is required. In recent years, targeted therapies such as ALK inhibitors and brentuximab vedotin (BV) have been developed. ALK inhibitors block the continuous activation of ALK kinase, a driver mutation that leads to cell proliferation in ALK-positive ALCL. Additionally, BV is an antibody–drug conjugate that targets CD30-positive cells. Both ALK inhibitors and BV have displayed dramatic effects in chemoresistant ALK-positive ALCL. Weekly vinblastine treatment and hematopoietic stem cell transplantation have also been reported to be effective therapies.
1. Introduction
Anaplastic large cell lymphoma (ALCL) is an aggressive T-cell lymphoma characterized by large T-cells with strong CD30 expression [
1]. Moreover, ALCL accounts for 10–15% of pediatric non-Hodgkin’s lymphoma cases and was first described in 1985 by Stein et al. [
2]. ALCL is classified into two subtypes based on anaplastic lymphoma kinase (ALK) expression: ALK-positive ALCL and ALK-negative ALCL [
3]. In pediatric patients, ALK-positive ALCL accounts for 80–100% of the cases, with a male predominance, whereas ALK-negative ALCL is more common in adults [
1,
2,
4]. Although ALK-negative ALCL generally has a worse prognosis than ALK-positive ALCL in adults, pediatric patients with ALK-positive and ALK-negative ALCL have similar prognoses [
4,
5]. In more than 90% of patients with ALK-positive ALCL, a rearrangement of the
ALK gene was detected. Specifically, nucleophosmin (
NPM)-
ALK was present in 80% of the cases, whereas tropomyosin 3 (
TPM3)-ALK was present in 15% [
6,
7]. The
NPM-ALK fusion gene results from a chromosomal translocation, t(2;5)(p23;q35), which brings together the
ALK gene at 2p23 and the
NPM gene at 5q35 [
8]. Conventional chemotherapy is effective in treating ALK-positive ALCL and is associated with a favorable prognosis, with 5-year event-free survival rates (EFSs) ranging from 65 to 75% [
9]. However, about 25–35% of ALK-positive ALCL patients experience relapsed or refractory disease. In addition, certain subtypes, such as “small cell variant” and “lymphohistiocytic variant,” have chemoresistant characteristics and poor prognosis, with a 5-year EFS of approximately 50% [
10]. Relapsed or refractory ALK-positive ALCL is often resistant to conventional chemotherapy. Therefore, salvage therapy is required. In recent years, targeted therapies, such as ALK inhibitors and brentuximab vedotin (BV) have been developed, and they have demonstrated dramatic responses in chemoresistant ALK-positive ALCL [
11,
12,
13]. Additionally, hematopoietic stem cell transplantation (HSCT) has been reported as an effective therapy for relapsed or refractory ALK-positive ALCL [
14,
15,
16].
2. Clinical Features
ALK-positive ALCL typically manifests as a highly aggressive stage III to IV disease with systemic symptoms, especially high fever [
1]. Extranodal lesions are frequently observed in ALK-positive ALCL (60%), such as those affecting the skin (19–21%), bone (17–19%), soft tissues (16–17%), bone marrow (11–12%), lungs (11–21%), and liver (8–14%) [
4]. However, the involvement of the gut and central nervous system (CNS) is uncommon [
4]. Bone marrow involvement is defined as the existence of ALCL cells in bone marrow determined through the analysis of the bone marrow smears.
3. Oncogenic Mechanism
Rearrangements of the
ALK gene have been detected in more than 90% of patients with ALK-positive ALCL. The expression of the wild-type ALK protein is strictly restricted to a few scattered cells in the CNS from birth [
8]. Upon ligand binding, the wild-type ALK protein undergoes homodimerization, which activates tyrosine kinases in the intracellular tail of the ALK molecule. The NPM-ALK fusion protein consists of an NPM oligomerization domain and an intracytoplasmic ALK region, including the tyrosine kinase domain. The expression of the
NPM-ALK fusion gene is controlled by the
NPM promoter, resulting in the continuous production of the NPM-ALK protein [
17]. Homodimerization of the NPM-ALK protein by the NPM oligomerization domain activates ALK tyrosine kinase, leading to cell proliferation [
1]. Even in
TPM3 or tropomyosin-receptor kinase fused (
TFG) genes, which are other partner genes of the
ALK gene and include a dimerization domain, homodimerization of each fusion protein by dimerization domains contributes to the activation of ALK tyrosine kinase and oncogenic activity [
1].
4. Risk Factors with Poor Prognosis
Several risk factors associated with poor prognosis have been identified in pediatric ALK-positive ALCL. These include morphological patterns with the small cell variant or lymphohistiocytic variant, leukemic presentation, MDD, and CNS involvement [
18,
19].
4.1. Morphological Pattern
The common type of ALK-positive ALCL is characterized by large tumor cells with horseshoe-shaped nuclei, abundant cytoplasm containing numerous vacuoles, and strong positive staining for ALK and CD30 [
1]. The small cell variant is a subtype of ALK-positive ALCL characterized by the coexistence of mainly small tumor cells that stain negative or weakly positive for ALK and CD30, along with a minor population of large tumor cells that stain strongly positive for ALK and CD30 [
1]. Additionally, the small cell variant accounts for 6% of all ALK-positive ALCL cases [
20]. The small cell variant has been identified as a risk factor for relapsed or refractory diseases [
18]. Small tumor cells in the small cell variant have been reported to be more resistant to chemotherapy than large tumor cells [
21].
The lymphohistiocytic variant is characterized by numerous histiocytes surrounding CD30-positive large tumor cells [
1]. Immunohistochemistry revealed that the histiocytes were negative for the nuclear proliferation marker Ki-67, indicating that they had assembled reactively rather than were undergoing neoplastic proliferation [
1]. The lymphohistiocytic variant has been reported to be a risk factor for relapsed or refractory disease [
18].
4.2. Leukemic Presentation
Leukemic presentation is defined as the presence of circulating ALCL cells in peripheral blood and is associated with an extremely poor prognosis. Leukemic presentation is extremely rare, accounting for less than 5% of all ALCL cases, and 75% of patients with leukemic presentation have a small cell variant histology [
22]. Furthermore, most pediatric patients with leukemic presentation are associated with the
NPM-ALK fusion gene. Diffuse lung infiltrates, respiratory distress, and pleural effusion are characteristic clinical features of leukemic presentation and have been reported in 50% of patients [
22]. The diagnosis of ALK-positive ALCL with a leukemic presentation can be challenging because the condition mimics T-cell leukemia [
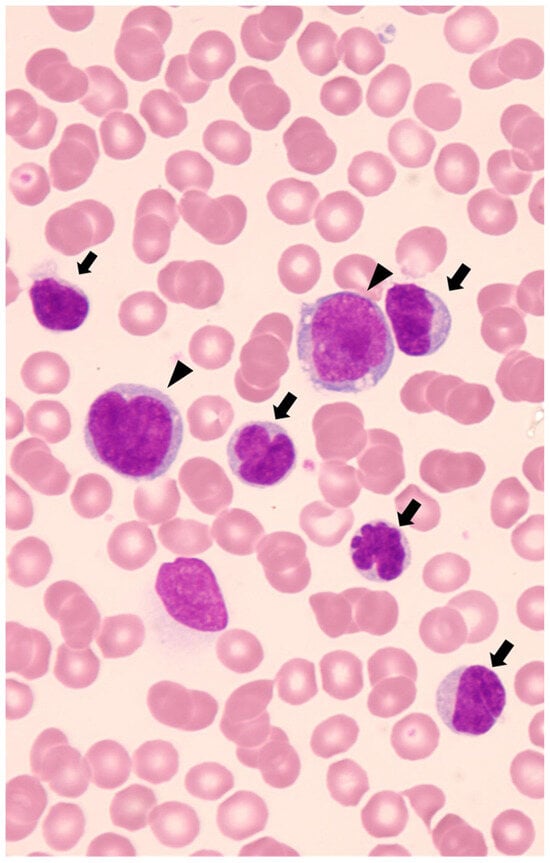
22]. Identification of lymphoma cells with characteristic karyomorphism, such as “flower-cell” or “cerebriform cell,” will contribute to the diagnosis of leukemic presentation. (
Figure 1) Moreover, when these characteristic cells present immunophenotypic findings as CD30- and ALK-positive, they can assist in the diagnosis of ALK-positive ALCL with a leukemic presentation [
22]. Notably, lymphoma cells in peripheral blood often express myeloid-associated antigens, including CD13, CD11b, and lysozyme [
22]. Whereas we encountered a patient with a leukemic presentation of a small cell variant whose circulating small tumor cells exhibited low expression of CD30 and ALK on immunohistochemistry and flow cytometry, with only one-tenth of
NPM-ALK mRNA expression compared to large tumor cells [
21]. This case highlights the importance of careful examination of cell morphology, even if CD30 and ALK expression in circulating tumor cells is low. Additionally, the lower percentage of tumor cells in the bone marrow than in the peripheral blood may contribute to the diagnosis of leukemic presentation [
23]. As leukemic presentation has a very poor prognosis, an accurate diagnosis is crucial for selecting effective salvage therapies.
Figure 1. May-Giemsa-stained blood smears at the onset. Numerous small-sized lymphocytes with lobulated nuclei called “flower-cell” or “cerebriform cell” (arrow), and a few large-sized lymphocytes with basophilic and vacuolated cytoplasm (arrowhead) are displayed.
4.3. Minimal Disseminated Disease
Minimal disseminated disease (MDD) is defined as a minimal lesion detected in the peripheral blood and/or bone marrow using PCR to target the
ALK-associated fusion gene. The
NPM-ALK fusion gene is commonly targeted in many reports [
18].
With regard to the PCR methods used to detect MDD, qualitative PCR (RT-PCR), RQ-PCR, and digital PCR (dPCR) have been reported. Damm-Welk et al. described that the sensitivity of RT-PCR was 10
−5 NPM-ALK-positive cells per control cells [
24]. In total, 48–61% of patients have a positive RT-PCR result in bone marrow, which indicates a poorer prognosis compared to those with a negative RT-PCR result [
23,
24,
25]. They reported that, in positive and negative RT-PCR, the cumulative incidence of relapse was 50% and 15%, 5-year EFS was 38% and 82%, and 5-year OS was 60% and 86%, respectively [
24]. The RQ-PCR method for
NPM-ALK detects each copy number of
NPM-ALK and internal control genes such as
ABL. Then, NPM-ALK copy numbers are normalized using the copy numbers of internal control gene. The normalized copy numbers (NCNs) have been defined as
NPM-ALK copy numbers per 10
4 copies of
ABL in several reports [
24,
26]. More than 10 NCNs are related to poor prognosis [
24,
26]. Damm-Welk et al. reported that in more than 10 NCNs and 10 or fewer NCNs in bone marrow, the cumulative incidence of relapse was 71% and 18%, 5-year EFS was 23% and 78%, and 5-year OS was 46% and 85%, respectively [
24].In recent years, MDD detection using dPCR has been reported [
27]. More than 30 NCNs have been linked poor prognosis [
27,
28]. A study by Damm-Welk et al. revealed that the 5-year progression-free survival (PFS) was 35%, 69%, and 74% for patients with more than 30 NCNs, 30 or fewer NCNs, and negative in bone marrow, respectively [
27]. Although MDD quantification using the RQ-PCR method cannot be compared across different laboratories and international settings, the dPCR method demonstrates high inter-laboratory reproducibility [
27].
4.4. CNS Involvement
In pediatric patients with ALCL, CNS involvement is rare, occurring in only 2.6% of cases [
19]. Solitary ALCL localized in the CNS has been reported in only three cases [
19]. Recently, CNS involvement was identified as a poor prognostic factor. Patients with CNS involvement have a worse prognosis than those with common ALK-positive ALCL, with a 5-year overall survival (OS) of 74% and 5-year EFS of 50% [
19]. The lymphohistiocytic variant morphology and leukemic presentation have been reported as risk factors for CNS involvement. In particular, 36% of the patients with lymphohistiocytic variants developed CNS involvement [
23].
This entry is adapted from the peer-reviewed paper 10.3390/cancers15245733