Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Engineering, Electrical & Electronic
There has been significant progress in IoT solutions for a variety of fields. The real-time functionality and remote deployment of IoT solutions are two crucial aspects that are necessary for their successful implementation. To achieve this, external batteries play a major role. While lithium–ion batteries are often the go-to choice for IoT devices, it is essential to recognise that different IoT applications have unique needs. Therefore, it is important to conduct a thorough examination of existing battery solutions and their suitability for various IoT applications.
- battery
- Internet of Things (IoT)
1. Introduction
The Internet of Things (IoT) has ushered in an era of unparalleled connectivity, transforming everything from consumer gadgets to large-scale industrial systems [1]. In healthcare, for example, IoT-enabled medical devices monitor patient vitals in real time, potentially reducing hospitalisation rates [2,3,4]. In agriculture, IoT sensors track environmental conditions [5], optimising yield and conserving resources [6,7]. An overview of different possible IoT applications is illustrated in Figure 1. The key to the seamless operation of these diverse applications lies in their ability to deliver real-time functionality and to be remotely deployed—two factors that are pivotal for the successful implementation of any IoT system.
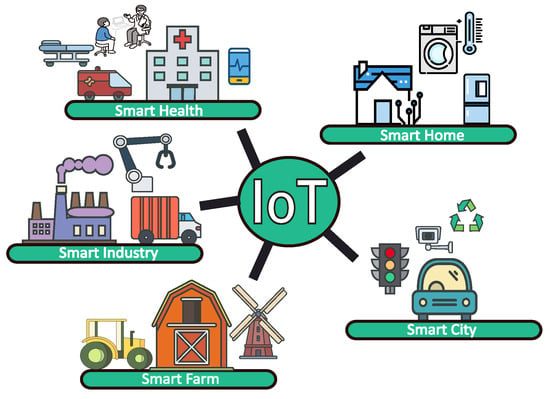
Figure 1. Different applications of Internet of Things.
If IoT is the engine driving the next wave of technological innovation, then batteries can be considered as the fuel. Due to the range of application requirements, IoT sensors often need to be run remotely for an extended period, making the choice of battery a crucial decision in the IoT system setup.
While lithium–ion batteries are often heralded as the default option for IoT applications, this one-size-fits-all approach overlooks the unique energy needs of different IoT environments. Just as the power requirements of a smartwatch differ from an industrial sensor in a factory, so too do the best-suited battery technologies. Therefore, it is crucial to scrutinise existing battery solutions and assess their compatibility with various IoT applications. Without such an examination, we risk limiting the efficacy and potential impact of IoT technologies, prompting the necessity for comprehensive research in this domain.
Many surveys have been conducted on the suitability of different types of lithium–ion-based batteries for specific applications such as electric vehicles [8,9,10,11]. However, there is little or no review work available on the suitability of different types of battery technologies for a wide range of IoT-based applications.
2. IoT Applications
IoT refers to a wide range of applications that use sensors, software, and network connectivity to collect and transmit data from everyday objects. This process improves operational efficiency and enhances user experience. IoT applications are becoming more diverse and widespread, and have established new possibilities for transformation in numerous fields. This work will focus on five IoT domains, namely healthcare, smart cities, smart homes, and farming and industrial automation.
2.1. Healthcare
The adoption of the Internet of Things (IoT), often known as the Internet of Medical Things (IoMT), has transformed the healthcare industry, allowing for more accurate diagnoses, personalised treatments, and better patient health outcomes. IoMT has assisted a diverse end-user group, including patients, healthcare providers, medical staff, and other closely related fields. The healthcare industry has improved by leveraging the application of IoT not just in patient-centric care to monitor patients remotely, provide personalised care, and optimise treatment plans but also in managing the hospital effectively, such as tracking inventory and equipment, monitoring patient safety, and managing hospital operations more efficiently and hence reducing healthcare costs [14]. Further analysis of the IoMT can be conducted in the sub-contexts of wearable and non-wearable applications.
Wearable
Wearable devices are a significant component of the IoMT ecosystem, particularly during health crises like the COVID-19 pandemic, where they played a crucial role in monitoring and managing health [15,16]. The smart, connected devices worn on the body, such as fitness trackers, smartwatches, and wearable monitors, gather information that is context-oriented and related to physical, behavioural, and psychological health [17,18,19]. These devices aid in the overall management of personal and community health, early detection of health issues, and timely intervention. However, with the increasing demand for wearable and implantable medical devices, there is an increasing need for reliable, safe, and long-lasting power sources.
Non-wearable IoT devices in healthcare are tools not worn on the body but which play a critical role in patient care. These devices, combined with the concept of IoT, offer efficient means for telehealth interventions, improving hospital efficiency, reducing human errors [20], and enhancing patient care. Examples include remote patient monitoring tools and medical dosage monitoring [20]. Hospital room automation, in-hospital contact tracing, medication management, patient safety, and more can be achieved through the integration of these non-wearable devices into the existing systems [21,22].
2.2. Smart Cities
In recent years, the concept of smart cities has gained significant traction owing to the advent of Internet of Things (IoT) solutions that aim to enhance the urban living experience and operational efficiency. IoT has enabled the development of various applications that can address urban challenges, such as real-time people counting and traffic monitoring systems to mitigate congestion and improve road safety [23]. Smart waste management systems can ensure timely trash collection and recycling, while IoT-enabled energy grids can optimize energy distribution and reduce wastage. Additionally, smart water systems can monitor and control water usage, aiding in the conservation of this vital resource. The immense potential of IoT in smart cities is evident, with each application contributing to a more sustainable and livable urban environment [24,25].
2.3. Smart Home
The domain of smart homes is centred around enhancing living comfort and security. Through IoT, homeowners can remotely control and monitor home appliances, security systems, lighting, heating, and cooling systems from anywhere. Applications like smart thermostats, automated locks, and voice-activated assistants have already found a place in many homes [26,27]. Furthermore, IoT can facilitate energy management through smart meters and appliances that operate during off-peak energy times to reduce electricity bills [28]. The development of more intuitive and integrated IoT applications continues to redefine the concept of home, making daily life more convenient and secure.
2.4. Smart Farm
The agriculture sector, too, stands to benefit significantly from IoT-powered smart farming. IoT applications in agriculture include precision farming, where sensors monitor soil conditions and crop health to provide actionable insights for farmers [29,30]. Automated irrigation systems can be developed to optimize water usage based on real-time data, while drone technology can aid in monitoring large farmlands and executing tasks like seeding or spraying pesticides [31]. Moreover, IoT can facilitate livestock monitoring through wearable devices that track the health and location of animals, ensuring their well-being and reducing losses [32].
2.5. Smart Industry
The transition of the industrial sector towards Industry 4.0 is largely driven by the adoption of IoT technologies. IoT applications in industries are vast and include the real-time monitoring of machinery to predict and prevent breakdowns, thus minimising downtime. Supply chain and inventory management can also be streamlined through IoT-enabled tracking systems. Additionally, smart safety gear can be developed to monitor the well-being of workers in hazardous environments, providing alerts in case of emergencies. These applications not only enhance operational efficiency but also contribute to creating safer and more sustainable industrial ecosystems.
3. Batteries for IoT Applications
3.1. Lead-Acid Batteries
Lead–acid batteries, invented in 1859, are still extensively used in the automotive industry [33]. The battery consists of acid and two lead electrodes, as seen in Figure 2. The lead–acid battery works by utilising a series of electrochemical reactions between its two primary electrodes which are a lead dioxide (PbO2PbO2) positive plate and a sponge lead (Pb) negative plate, both immersed in an electrolyte solution that is composed of sulphuric acid (H2SO4H2SO4) and water (H2OH2O). When the battery is discharged, the PbO2PbO2 and Pb plates react with the sulphuric acid to produce lead sulphate (PbSO4PbSO4) and water. This reaction releases electrons, thereby providing electrical power. On the other hand, during the charging process, the lead sulphate and water are converted back into PbO2PbO2, Pb, and sulphuric acid [34]. One of the challenges associated with this type of battery is sulphation. This happens when the battery remains discharged for an extended period, leading to the formation of larger, less reversible lead sulphate crystals. Lead–acid batteries are known for their cost-effectiveness and reliability, but their energy density is lower than many modern battery technologies.
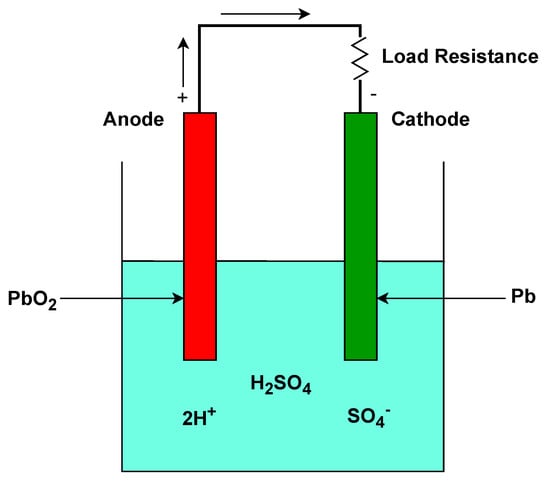
Figure 2. Operational setup of lead−acid battery.
3.2. Nickel–Metal Hydride (NiMH) Batteries
Developed in the 1980s as a successor to nickel–cadmium batteries, nickel–metal hydride (𝑁𝑖𝑀𝐻) batteries are commonly used for consumer electronics, power tools, and hybrid electric vehicles [35]. The electrochemical reactions (Figure 3) between the primary components of 𝑁𝑖𝑀𝐻 batteries, the positive electrode made of nickel hydroxide (Ni(OH)2Ni(OH)2), and the negative electrode, a metal alloy capable of forming hydrides, enable their operation. During discharging, the nickel hydroxide at the cathode oxidises to nickel oxyhydroxide and releases electrons, while the metal alloy at the anode reacts with hydroxide ions to form a metal hydride. When charging, these reactions reverse. Compared to their predecessor, nickel–cadmium (𝑁𝑖𝐶𝑑) batteries, 𝑁𝑖𝑀𝐻 batteries have a higher energy density, a lower “memory effect” (wherein batteries lose capacity if not fully discharged), and a more environmentally friendly composition since they lack the toxic cadmium. While superior to 𝑁𝑖𝐶𝑑, they exhibit a higher self-discharge rate and lower energy density than contemporary lithium–ion batteries. 𝑁𝑖𝑀𝐻 batteries are ideal for various applications due to their reliability, size versatility, and relatively lower cost than other types of batteries [36].
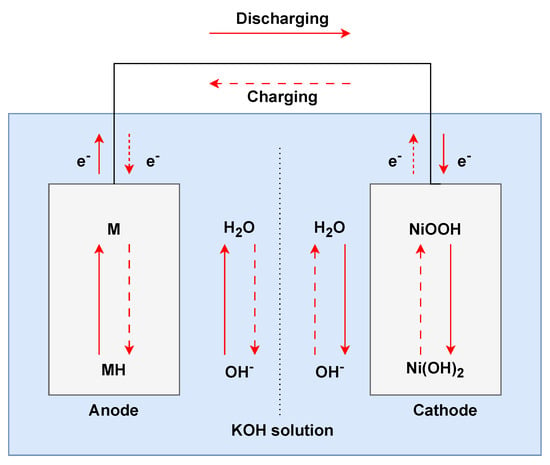
Figure 3. Operational setup of nickel−metal hydride battery.
3.3. Lithium–Ion (Li–Ion) Batteries
The lowest reduction potential and highest cell potential makes the element lithium a popular choice for batteries [37,38]. Lithium–ion batteries are a popular choice of battery over a wide range of applications ranging from portable electronics to electric vehicles. This is mainly due to their energy density, low self discharge, and low memory effect when compared to the other secondary (rechargeable) batteries.
In the 1970s, the development of lithium-based rechargeable batteries marked a significant milestone in the energy industry. In the following decade, the introduction of oxide cathodes further enhanced the performance of these batteries. The researchers later shifted their focus towards improving cell voltage and stabilisation, later leading to the commercialisation of rechargeable batteries by Sony in 1991. Since then, these batteries have become increasingly popular in the market and are widely used in various applications [39].
Lithium–ion cells are made up of three main components: a cathode composed of lithium metal oxide, an anode made from graphite, and an electrolyte that contains lithium ions. When the battery discharges, the lithium ions move from the anode to the cathode through the electrolyte, while electrons flow through the external circuit, providing power to the device. This process is reversed during charging [40].
Compared to traditional batteries, the unique structure of lithium–ion batteries provides an advantage due to their ability to generate electricity without dissolving the electrodes in the electrolyte. This is possible due to the presence of lithium in the cathode and its efficient storage capability in the carbon anode. This process helps prevent battery deterioration and increases the number of charge and discharge cycles the battery can undergo.
The lithium–ion batteries can be further divided into the following subcategories based on the cathode material and are summarised in Figure 4.
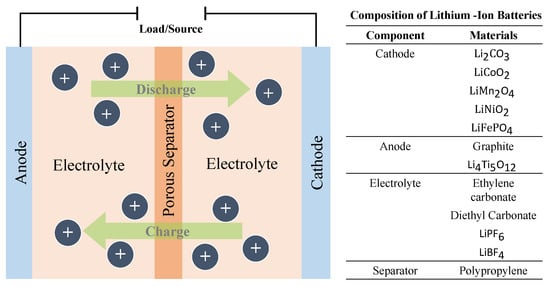
Figure 4. Operational setup of lithium–ion battery.
3.3.1. Lithium Cobalt Oxide (LCO) Batteries
Lithium cobalt oxide (LiCoO22) is a widely used cathode material in rechargeable lithium–ion batteries due to its high energy density. The material has a layered structure that is easy to synthesise and handle, making it a popular choice for commercial applications. However, cobalt is a scarce and expensive resource, which can limit the production of LiCoO22 batteries. Additionally, LiCoO22 batteries have a relatively short lifespan and can suffer from thermal instability, increasing safety concerns. Furthermore, the battery exhibits low specific power, which can limit its ability to deliver high power output. Despite these limitations, LiCoO22 batteries remain popular for applications wherein high energy density is a primary concern, such as in consumer electronics and electric vehicles [41].
3.3.2. Lithium Manganese Oxide (LMO) Batteries
Lithium manganese oxide (LiMn22O44) with a three-dimensional spinel structure is used as a cathode. The spinel structure allows for high thermal stability and improves ion flow on the electrode. Manganese lithium–ion batteries can produce the same voltage as cobalt lithium–ion batteries and have the advantage that they can be made at a low cost.
However, there is a disadvantage to using manganese in lithium–ion batteries. During charging and discharging, manganese may dissolve out into the electrolyte, which can shorten the battery life. Therefore, researchers and manufacturers are continually working to improve the stability of the cathode material to overcome this issue and make lithium manganese oxide a more reliable and long-lasting option for lithium–ion batteries [42].
3.3.3. Lithium Nickel Manganese Cobalt Oxide
Lithium Nickel Manganese Cobalt Oxide (LiNiMnO22) is a type of cathode material that is a combination of nickel, manganese, and cobalt and combines the advantages of the earlier versions of cathode materials. This material has a high energy density and good stability, making it a popular choice for use in electric vehicles and other high-performance applications. Additionally, LiNiMnO22 has a relatively low cost compared to other cathode materials, making it an attractive option for large-scale battery production [43,44].
3.3.4. Lithium Iron Phosphate (LiFePO44) Batteries
Lithium iron phosphate (LFP) is a type of lithium–ion battery that uses lithium iron phosphate as the cathode material. The olivine structure of LiFePO44 gives LFP batteries their distinct electrochemical properties. The one-dimensional channels in the olivine structure allow for the relatively easy migration of lithium ions during the electrochemical processes. However, these channels also limit the rate at which these processes can occur, resulting in lower power densities compared to other lithium–ion chemistries. One of the significant environmental benefits of LFP batteries is the absence of heavy metals like cobalt and nickel in their cathode material. This makes them more environmentally friendly compared to other lithium–ion chemistries. LFP batteries have a higher cycle life compared to other lithium–ion chemistries, making them suitable for applications wherein frequent charging and discharging are required. Additionally, the thermal stability of the FePO44 cathode material reduces the risk of thermal runaway, making LFP batteries one of the safest lithium–ion chemistries available [45].
3.3.5. Lithium–Polymer (LiPo) Batteries
The ideation of a battery with the potential to eliminate liquid electrolytes and, consequently, minimise the risk of leakage and safety hazards, has led to the development of lithium–polymer (LiPo) batteries. LiPo batteries offer a flexible and lightweight alternative to conventional lithium–ion batteries, making them commercially viable. The polymer in the LiPo batteries can be either “dry” or “gel-like”, facilitating the movement of lithium ions between the cathode and anode [46,47].
3.4. Solid-State Batteries
The popularity of lithium batteries has increased significantly, but they come with significant challenges. The use of liquid electrolytes makes them highly volatile and flammable outside their operating temperature, which raises safety concerns. The limited ionic conductivity and electrochemical stability also limit the cells’ voltage and capacity, thereby decreasing the energy density of lithium–ion batteries. However, solid-state electrolytes (SEs) can be an effective alternative to liquid electrolytes and can achieve much higher energy density and safety, while also overcoming most of the drawbacks of Li–ion batteries (LIBs) [48]. The lithium ceramic batteries are a promising subset of solid-state batteries that have a ceramic electrolyte. This ceramic material can conduct lithium ions between the cathode and anode during the battery’s charge and discharge cycles [49,50].
3.5. Alkaline Batteries
The alkaline battery was introduced in the 1960s as a significant improvement over the zinc–carbon batteries that came before it. Its primary use is still in consumer electronics, such as remote controls, flashlights, toys, and digital cameras. Alkaline batteries have become popular due to their relatively high energy density, longer shelf life, and consistent voltage output compared to zinc–carbon batteries. They work by electrochemical reactions between the manganese dioxide (MnO22) cathode and the zinc (Zn) anode, which are immersed in an alkaline electrolyte, typically potassium hydroxide (KOH) (Figure 5). When the battery discharges, zinc at the anode undergoes oxidation, releasing electrons and producing zinc oxide. At the same time, manganese dioxide at the cathode is reduced, consuming electrons. This flow of electrons from anode to cathode produces electrical power. Unlike other battery types, alkaline batteries are primary cells, which means they are not rechargeable; once the chemicals inside are exhausted, the battery is depleted. Alkaline batteries have potential for IoT applications that require infrequent battery replacement or wherein device simplicity and low cost are paramount. Due to their stable voltage output and long shelf life, alkaline batteries can be ideal for low-drain IoT sensors or devices that remain in a standby state for a long time and only transmit data from time to time. Moreover, their widespread availability and standardised sizes, such as AA and AAA, make them convenient for consumer-facing IoT products, allowing users to replace batteries quickly [51].
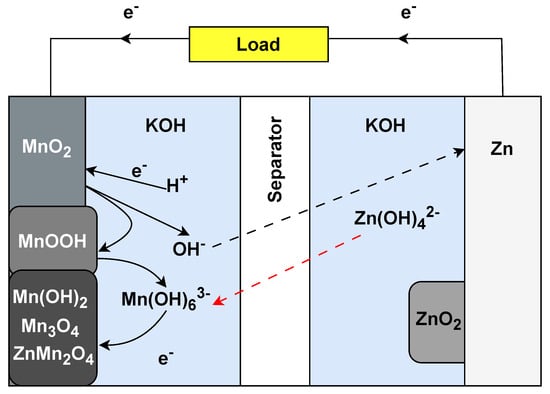
Figure 5. Operational setup of alkaline Zn−MnO22 battery.
3.6. Zinc–Air Batteries
Designed initially as non-rechargeable batteries, zinc–air batteries gained popularity in the late 20th century due to their high energy density. It was an attractive choice for certain applications, particularly for small devices such as hearing aids. A zinc–air battery (Figure 6) comprises a zinc anode, an air (oxygen) cathode, and an alkaline electrolyte. The air cathode enables oxygen from the surrounding air to take part in the electrochemical reactions and helps in the electrons’ movements. One key drawback of zinc–air is its short cycle life [52]. Zinc–air batteries are ideal for long-term low power and remote sensing applications as they can remain inactive for extended periods and provide power as required [53,54,55].
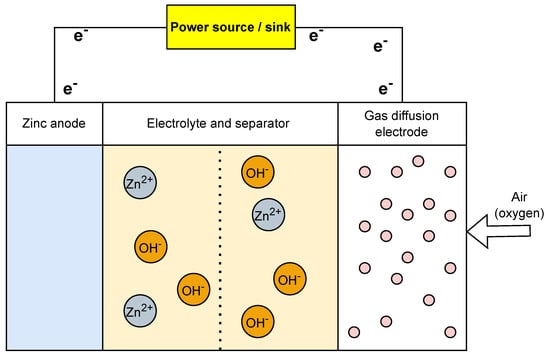
Figure 6. Operational setup of zinc−air battery.
3.7. Flow Batteries
Flow batteries, also known as redox flow batteries, were introduced in the 1970s, but their popularity as a solution for large-scale energy storage only became prominent in recent years. These batteries are primarily used in grid energy storage due to their ability to store vast amounts of energy for long periods. This makes them an ideal choice for integrating renewable energy sources such as solar and wind into the power grid as they can store excess energy generated during peak times and release it when the demand is high or generation is low. Flow batteries (Figure 7) work based on the electrochemical reactions of two liquid electrolyte solutions separated by a membrane. These electrolytes are stored in external tanks and are pumped through a cell stack, where they undergo redox reactions. In the cell stack, one electrolyte is oxidised at the negative electrode, releasing electrons, while the other is reduced at the positive electrode, accepting those electrons to generate electricity. One of the most significant advantages of flow batteries is their energy capacity, which is determined by the size of the electrolyte tanks. On the other hand, the size and design of the cell stack determine the power output, i.e., how quickly energy can be delivered, making them highly scalable and customisable based on specific requirements. While flow batteries are primarily designed for large-scale applications, they can also be used in IoT scenarios where long-duration, uninterrupted power is essential. For instance, in remote IoT sensor networks that monitor environmental parameters or infrastructure over extended areas, a flow battery system can ensure a consistent power supply, especially when combined with renewable energy sources. Flow batteries are also suitable for applications wherein maintenance or battery replacement is challenging due to their long cycle life and ability to undergo deep discharges without significant degradation. However, the requirement of external tanks for electrolytes and their complexity makes miniaturisation a challenge, making them less suitable for compact IoT devices [56].
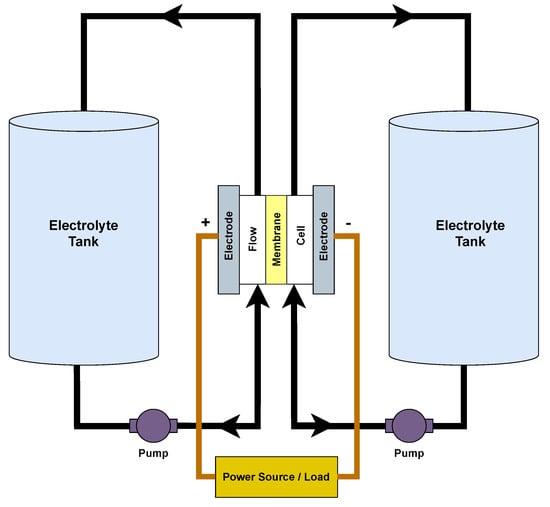
Figure 7. Operational setup of redox flow battery.
3.8. Supercapacitors
Supercapacitors have emerged as promising energy storage components in recent years. They are particularly useful in hybrid configurations where they are combined with other energy storage devices to improve overall performance. Supercapacitors are also called electric double-layer capacitors (EDLCs) or electrochemical capacitors (ECs), and they work by storing electrical energy in the double layer between the electrodes and the electrolyte. Supercapacitors offer higher specific energy than traditional capacitors and greater specific power than existing batteries. As a result, they are ideal for applications that require short charge–discharge cycles, ranging from a few seconds to several minutes [57,58].
However, it is essential to acknowledge the current limitations in the technological maturity of supercapacitors in the domain of IoT applications. As of now, their integration as standalone energy storage solutions in IoT systems remains largely theoretical and requires further research and development.
This entry is adapted from the peer-reviewed paper 10.3390/batteries9120580
This entry is offline, you can click here to edit this entry!
